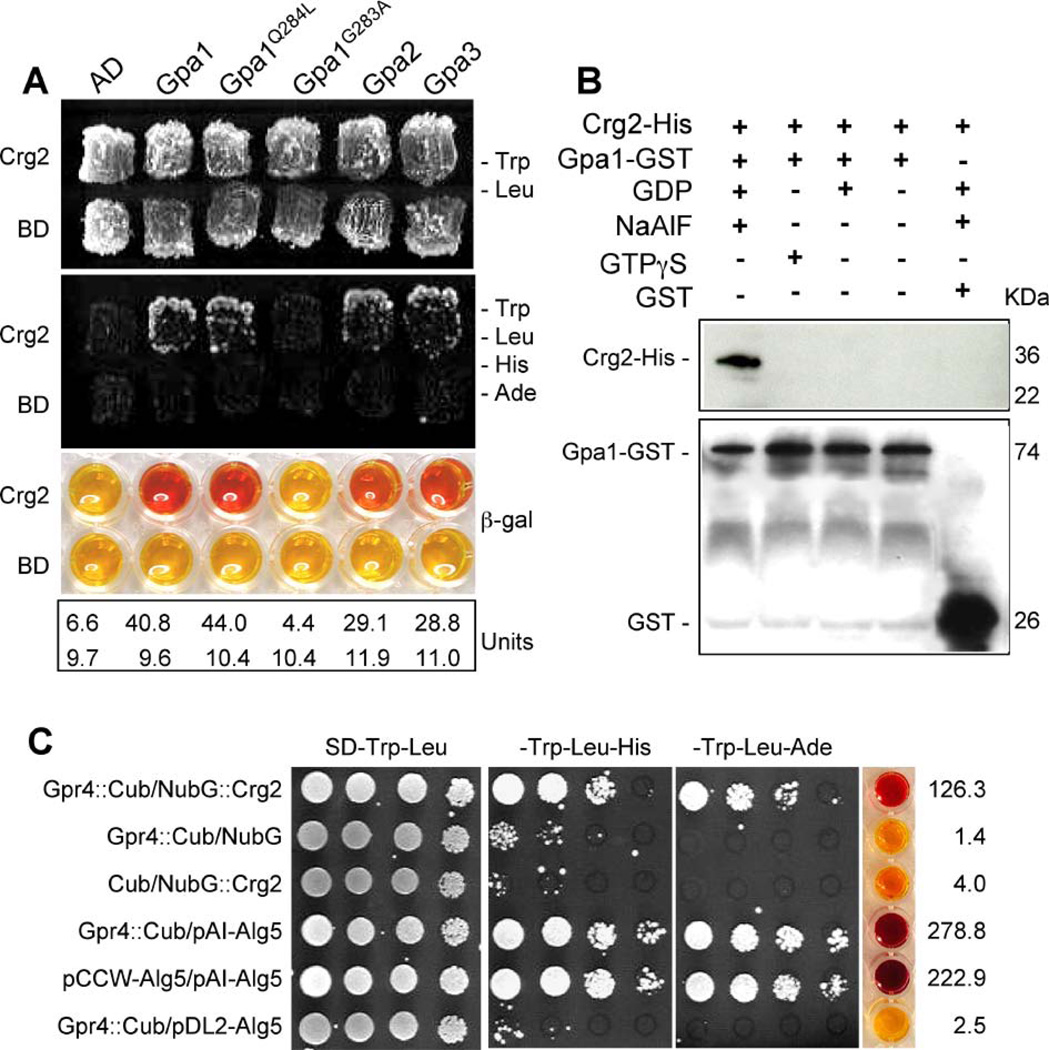
Figure 4. Crg2 interacts with three Gα subunits.
A) Crg2 interacts with both Gpa1 and a Gpa1 dominant active mutant in the yeast two-hybrid interaction assay. The first 910 bp of the CRG2 cDNA was fused with the activation domain (AD), while the full-length cDNA of GPA1 and GPA1Q284L was fused with the binding domain (BD). Both fusion constructs were introduced into the yeast host strain PJ69-4A and colonies grown on SD medium without tryptophan and leucine were tested on medium also lacking histidine and adenine. β-galactosidase enzyme activity was measured for each strain. B) The interaction between Crg2 and Gpa1 was verified in an in vitro binding assay. Both Crg2-His fusion protein and Gpa1-GST fusion protein were expressed and purified from E. coli strain DL21 (DE3). Gpa1-GST protein extract was mixed with glutathione agarose to immobilize the Gpa1-GST protein. Crg2-His protein extract was added to the washed Gpa1-GST-agarose and the in vitro binding assay between the two tagged proteins was performed by incubating at 4°C for 1 h in the presence of NaAlF and GDP, GTPγS, GDP, or no nucleotide. After washing the agarose, protein mixtures were eluted and loaded on SDS-PAGE gel. Western blot was performed with His antibody (upper panel) and GST antibody (bottom panel). C) Interactions between Crg2 and Gpr4 were observed in the split-ubiquitin system. The C-terminal half of ubiquitin (Cub) was fused to the C-terminus of Gpr4 cDNA (Gpr4::Cub). The N-terminal half of ubiquitin (NubG) was fused to the N-terminus of Crg2 cDNA (NubG::Crg2). Gpr4::Cub interaction with the control vector pAI-Alg5 served as a control to ensure the correct topology of the Gpr4::Cub fusion protein. Gpr4::Cub interaction with the empty vector pDSL-NX and pDL2-Alg5, NubG::Crg2 interaction with empty vector pCCW served as negative controls, and pAI-Alg5 interaction with pCCW-Alg5 served as a positive control. β-galactosidase activity assays were performed to further verify the interactions. Each number in units was averaged from two independent experiments.