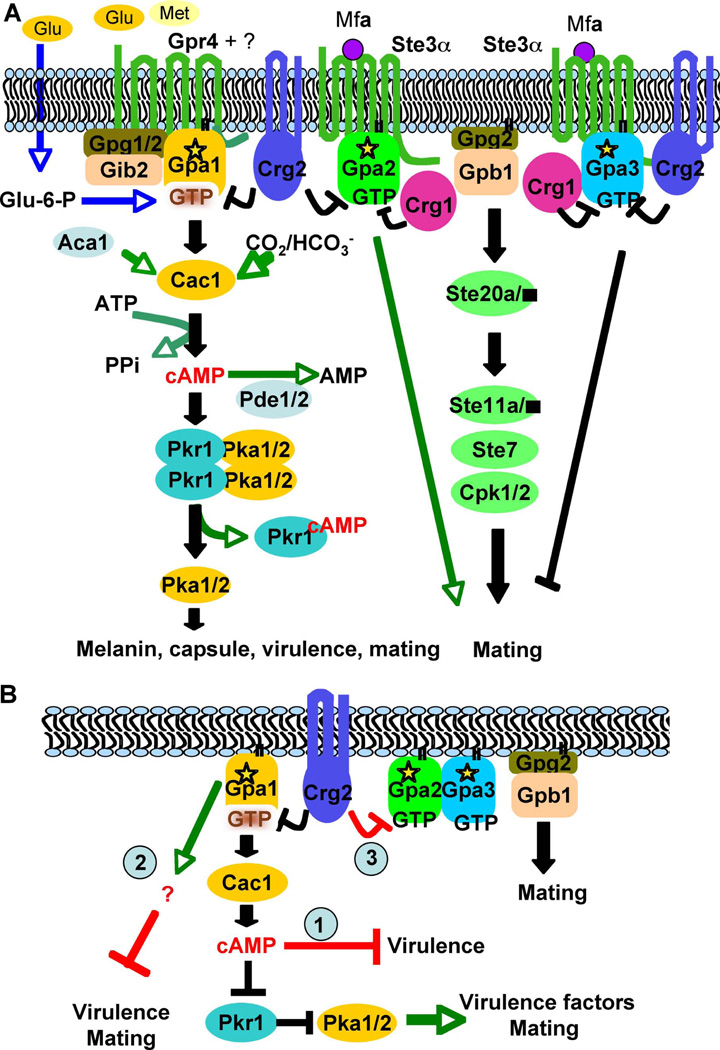
Figure 8. Model for the involvement of Crg2 in G protein complex regulation.
(A) In this model, the Gpr4 receptor activates Gpa1 and Crg2 functions as an RGS protein regulating Gpa1 inactivation. Crg2, Gpa1, and Gpr4 form a functional protein complex. Gib2 and Gpg1 function as βγ subunits that interact with the Gpa1 Gα subunit to govern Gpa1-cAMP signaling. Upon activation, the expression of Gpa2 is induced, and Gpa2 controls signaling by binding and releasing the Gβγ subunits. The active form of Gpa2 also plays a positive role in pheromone response that leads to mating, whereas the activated form of Gpa3 inhibits mating. Both Crg1 and Crg2 interact with the pheromone receptor Ste3 and the Gα subunits Gpa2 and Gpa3, and function to constrain Gpa2 and Gpa3 signaling by stimulating GTPase activity. (B) Three possible models on how Crg2 may be involved in the control of virulence. Model 1, crg2 mutants and GPA1Q284L expressing strains overproduce intracellular cAMP, which causes yeast cells to become more sensitive to stress responses than wild type and thus exhibit altered fitness in the host environment and overwhelm the impact of Pka1 activity. Model 2, the Ga protein Gpa1 also activates an alternative downstream signaling cascade in addition to the Cac1-cAMP-Pka1 signaling cascade, and this alternative target inhibits virulence and mating. Gpa1 activates both targets to maintain yeast cells in dynamic balance between virulence and mating. Model 3, interactions between Crg2 and Gpa2 and Gpa3 could also contribute to virulence.