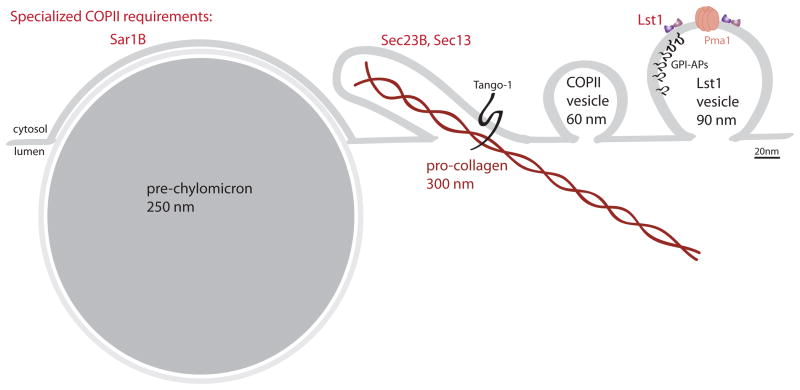
Figure 2. Flexible form and function of COPII vesicles.
The canonical COPII vesicles, as initially characterized in yeast, is 60–80nm in diameter and forms through the action of the minimal COPII coat, Sar1/Sec23/Sec24/Sec13/Sec31, on both complex ER membranes and synthetic liposomes. Different cargo molecules dictate distinct requirements for the size and shape of vesicles. Although the precise mechanisms by which these distinct morphologies are created, in many cases they require either specific COPII paralogs (noted in red) or additional cargo adaptors. In yeast, GPI-anchored proteins (GPI-APs) and the multimeric Pma1 complex depend on the Sec24 paralog, Lst1/Sfb2, for efficient capture into vesicles that are markedly larger than standard COPII vesicles. Pro-collagen assembles into long rods that are too long for a standard vesicle and are likely packaged into a more tubular structure, although these carriers have not been directly visualized. Efficient ER export of pro-collagen relies on the putative adaptor protein, TANGO-1, and is sensitive to mutations in SEC23B and knock-down of SEC13. Pre-chylomicrons are large lipid particles that accumulate in the ER when SAR1B is mutated.