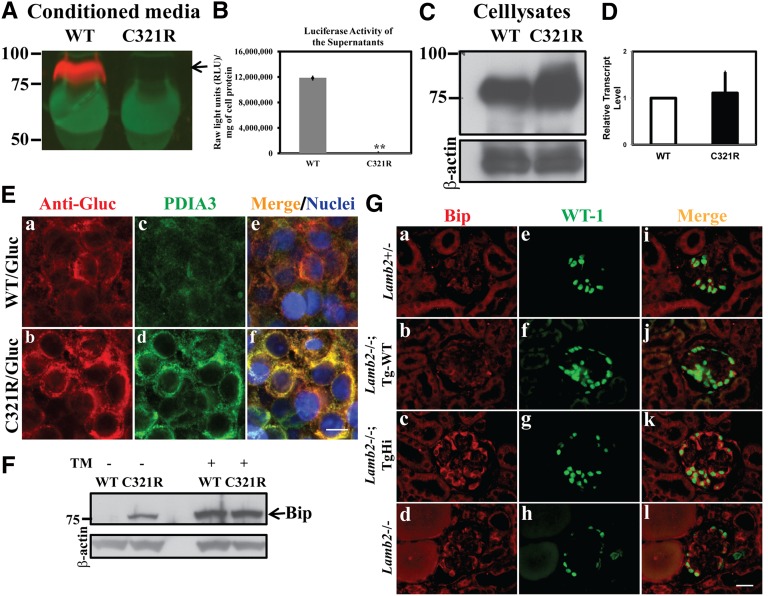
Figure 6.
Secretion of the mutant laminin β2 fragment/Gluc fusion protein is inhibited and podocyte ER stress is induced in TgHi Lamb2−/−; C321R-LAMB2 mice. (A, arrow) The WT but not the C321R-LAMB2/Gluc fusion protein was secreted from stably transfected HEK293T cells. (B) This finding was reflected by luciferase activity in the media (**P<0.001 by t test). The data are presented as mean ± SD of three independent media samples. (C) The C321R/Gluc fusion protein was retained intracellularly compared with the WT fusion protein. (D) Quantitative RT-PCR did not show a significant difference in Lamb2 mRNA levels between the WT and C321R cell clones. Data are presented as mean ± SD of fold changes of four independent samples from each clone. P>0.05 by t test. (E) Colocalization of the (a) WT/Gluc or (b) C321R LAMB2/Gluc fusion protein with the (c and d) ER marker PDIA3 in the 293T-Gluc cells using confocal microscopy. (e and f) Nuclei were counterstained with Hoechst 33342 (blue). (a, c, and e) The WT fusion protein seemed to be transiently associated with PDIA3, but (b, d, and f) the mutant was completely colocalized with PDIA3, indicating accumulation in the ER. (c and d) The mutant protein also induced expression of PDIA3. Scale bar, 10 µm. (F) Cell lysates of 293T-Gluc clones were subjected to Western blot analysis. The C321R but not the WT fusion protein increased BiP expression (arrow). Tunicamycin (TM) -treated cells served as positive controls. (G) Frozen kidney sections from mice of the indicated genotypes were examined by dual immunofluorescence staining of BiP and WT-1 at 3 weeks. Compared with the very low level expression of (a, e, and i) BiP in the control podocytes, BiP upregulation was detected in podocytes of (c, g, and k) TgHi glomeruli and (d, h, and l) some Lamb2−/− glomeruli but not (b, f, and j) podocytes of Tg-WT glomeruli. Scale bar, 20 µm.