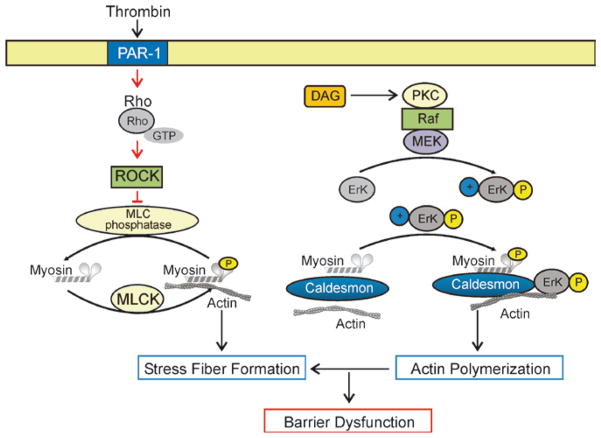
Fig. 27.5.
Endothelial barrier-regulatory mechanisms: role of MLC and caldesmon. Pulmonary endothelial cells (ECs) are characterized by a thin cortical actin ring with associated cell–cell (adherens junctions and cell–matrix or focal adhesions) connections that provide the structural framework for barrier integrity. Thrombin, a known barrier-disruptive agent, results in rapid recruitment of its receptor (PAR-1) into membrane lipid rafts. For activation of PAR-1 receptor, signaling and subsequent activation of the small guanosine triphos-activation is coupled to Gi phatase (GTPase) Rho. Rho stimulates rapid translocation of the actin-binding protein cortactin to the cell periphery, where it interacts with the barrier regulatory enzyme, myosin light chain kinase (MLCK). Phosphorylation of either of these proteins increases their interaction to actin in favor of stress fiber formation. Diacylglycerol (DAG) activates PKC, which activates Raf/MEK/ERK kinase activity. Erk phosphorylation subsequently facilitates the cytoskeletal caldesmon activation and phosphorylation, a key event for actin binding, polymerization, and fiber formation. This sequence of events occurs within minutes of stimulation and results in dramatically increased endothelial dysfunction. These pathways warrant evaluation of a potential therapeutic intervention with PTK such as sorafenib or statins for pulmonary hypertension