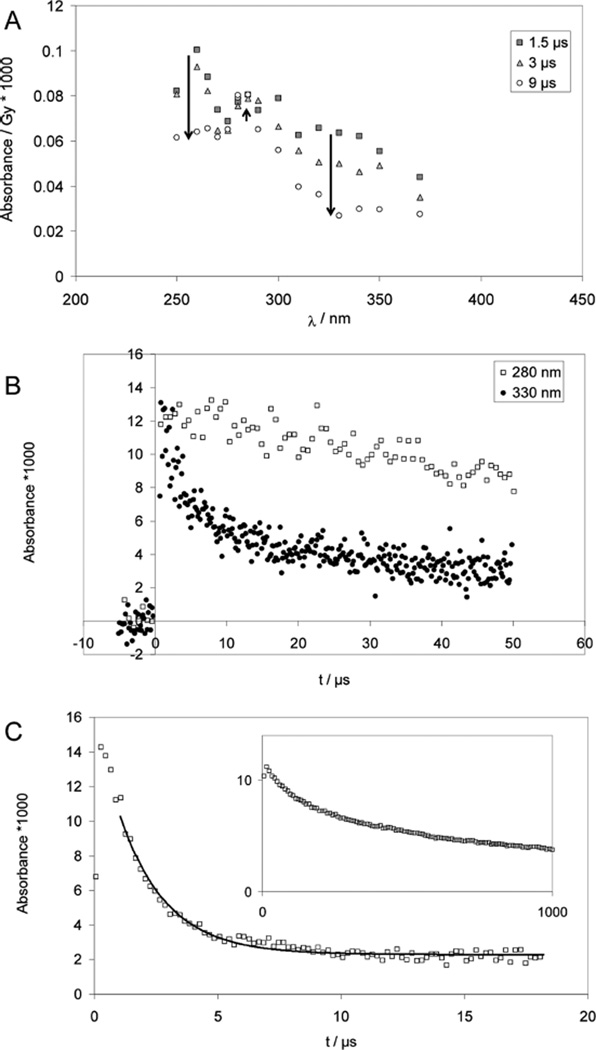
Figure 3.
Cysteine (CysSH) and cystine (CysSSCys). Panels A and B: Pulse radiolysis of Ar-saturated solutions of 1 mM CysSSCys, 10 mM H2SO4, and 2 M t-BuOH. Samples were irradiated with very high doses of approximately 150 Gy. Panel A: Spectra are shown for 1.5 µs (squares), 3 µs (triangles) and 9 µs (circles) after pulse. The bands at 330 nm (thiyl radicals) and 265 nm (•Cα radicals) decay quickly while a band at 280 nm (α-mercaptoalkyl radicals) forms. Panel B: Absorption-vs-time profiles at 280 nm (open squares) and 330 nm (filled squares); dose: approximately 150 Gy. Panel C: Pulse radiolysis of Ar-saturated solutions of 1 mM N-Ac–Cys–OMe, 10 mM KOH, 0.1 M t-BuOH; dose =21 Gy. The thiyl radical concentration is monitored via the equilibrium to the disulfide radical anion at 420 nm; the solid line represents a first-order fit, which yields kobs = 1 × 105 s−1. Inset: decay of the carbon-centered radicals monitored at λ = 330 nm; dose =25 Gy.