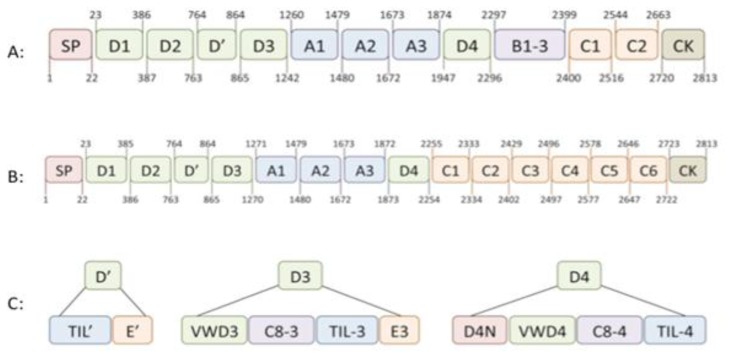
Figure 1. Domain structure of VWF.
The molecular architecture of VWF is characterized by the presence of distinct domain structures. Panel A represents the arrangement of five different structures according to the original analysis of the VWF sequence (reviewed by Pannekoek & Voorberg).38 The numbering of the domain boundaries has been used in our laboratory in the previous years. Panel B shows the domain organization as has recently been proposed by Zhou et al.41 One striking difference with the original domain structure is the replacement of the B1-3 - C1 - C2 domain region by 6 homologous C-domains. In addition, their analysis revealed that the D-domains consist of various independent structures, which are highlighted in panel C. The D1, D2 and D3 domains each contain a VW-domain, a trypsin inhibitor-like (TIL)-structure, a C8 fold and an E module. The D′ region lacks the VW domain and TIL-structure. The D4 domain lacks the E module, but instead comprises a unique sequence designated D4N.