Abstract
The effect of selective serotonin receptor inhibitors (SSRIs) on healthy individuals remains unclear. The aim of the trial was to evaluate the effect of the SSRI escitalopram on cognitive function in healthy first-degree relatives of patients with major depressive disorder (FDRs). A total of 80 FDRs were randomized to escitalopram (10 mg/day) (n = 41) versus placebo (n = 39) for 4 weeks. Neuropsychological tests and ratings of mood were applied at entry (T0) and at 4 weeks (T4). The main outcome measure was calculated as the change (T4–T0) in a general cognition score, which was the standardized mean of 13 test measures. Mean change in the general cognition score was not significantly increased with escitalopram compared with placebo (p = 0.37) or for any of the specific tests. In univariate analyses no statistically significant correlations were found between change in the general cognitive score and the variables age, sex, Hamilton depression score 17 items, Danish Adult Reading Test-45, and plasma escitalopram levels, respectively. These results suggest that treatment with escitalopram does not improve or impair cognitive function in FDRs. Improvement in cognitive function following treatment of depressed patients with SSRIs seems to be related to the effects on depressive symptoms rather than to a direct effect of the SSRI.
Keywords: cognitive function, escitalopram, healthy, high risk, major depressive disorder, placebo-controlled, trial
Introduction
In depression a wide range of cognitive deficits is a consistent finding [Ravnkilde et al. 2002]. Cognitive function is a predictor of the functional and psychosocial burden of illness in major depressive disorder (MDD) and consequently a pertinent candidate predictor of treatment response [Austin et al. 2001]. With recovery from MDD, abnormalities in cognitive function tend to normalize but cognitive impairment is also seen in recovered patients [Hasselbalch et al. 2010; Kessing, 1998]. Cognitive impairment has been reported in healthy first-degree relatives of patients with MDD, thus in a cross-sectional high-risk case–control study of healthy twins with and without a co-twin history of affective disorder, the healthy twins discordant for unipolar disorder showed lower performance on almost all measures of cognitive function: selective and sustained attention, executive function, language processing and working and declarative memory, and also after adjustment for demographic variables, subclinical affective symptoms and other minor psychopathology [Christensen et al. 2006]. Further, decreased immediate recall and recognition memory has been found in young women with no personal history of depression but with a depressed parent as compared with an age-matched control group with no family history of depression [Mannie et al. 2009].
Previous trials investigating the effect of selective serotonin receptor inhibitors (SSRIs) on cognitive function in healthy individuals have given inconsistent findings. In a recent review, concerning the effect of SSRIs in healthy individuals, 18 randomized trials using 39 different neuropsychological tests to investigate cognitive function were identified [Knorr and Kessing, 2010]. Treatment with a SSRI was found to improve [Murphy et al. 2008; Loubinoux et al. 2005; Harmer et al. 2004; Schmitt et al. 2001; Knutson et al. 1998, 1997], deteriorate [Riedel et al. 2005; Schmitt et al. 2002a, 2001; Fairweather et al. 1997; Ramaekers et al. 1995; Robbe and O'Hanlon, 1995] or have no effect on cognitive function [Peran et al. 2008; Paul et al. 2007, 2002; Wingen et al. 2006, 2005; Loubinoux et al. 2005; Riedel et al. 2005; Siepmann et al. 2003; Schmitt et al. 2002a, 2002b, 2001; Wilson et al. 2002; Allen et al. 1988; Fairweather et al. 1997; Ramaekers et al. 1995; Robbe and O'Hanlon, 1995]. It was concluded that the diverging findings could be a result of a number of methodological drawbacks. In general, no trial fulfilled the principles of conducting and reporting randomized trials according to the Consort Statement guidelines and the majority of the trials included a mix of healthy individuals with and without a family history of affective disorders [Knorr and Kessing, 2010]. More specifically, three smaller trials have investigated the long-term effect on cognitive function of intervention of escitalopram compared with placebo in healthy individuals. Two of these studies found no significant effect of escitalopram. Wingen and coworkers [Knorr and Kessing, 2010; Wingen et al. 2005, 2006] investigated doses of escitalopram 10–20 mg/day versus placebo for 15 days in a crossover design in 18 participants with an unknown family history of depression and found no effect on actual driving performance, psychomotor performance or visual memory performance. Paul and colleagues [Paul et al. 2007] investigated escitalopram 20 mg/day versus placebo for 14 days in a crossover design in 24 participants with an unknown family history of depression and found no effect on psychomotor performance evaluated by multiple tests. In the third and most recent trial, Drueke and colleagues [Drueke et al. 2009] administered 10 mg of escitalopram for a period of 7 days in a crossover design to 20 healthy male participants with no family history of major mental disorder suggesting a differential effects of serotonergic manipulation depending on whether the test was applied for the first or the second time
The diversity of symptoms in MDD suggests that many areas of the brain are involved in the pathophysiology of the disorder. The serotonin transporter is expressed abundantly in the raphe nucleus and in the limbic system which may be the main site of action for SSRIs [Sierksma et al. 2009]. It is, however, not clear whether treatment with SSRIs results in a direct improvement of cognition or whether the effect of SSRIs on cognitive function is secondary to the effect of SSRIs on depressive symptoms. A neuropsychological hypothesis of antidepressant drug action suggests that, at the neuropsychological level, antidepressants work by remediating negative affective biases in depression and anxiety and that these actions occur relatively quickly following drug administration [Harmer et al. 2009a, 2009b; Miskowiak et al. 2007]. To disentangle the effect of antidepressant treatment from the effect of recovery from the depressive disorder per se, we investigated the effect of a SSRI on cognitive function in healthy first-degree relatives of patients with MDD. As revealed previously, such individuals may present with cognitive disturbances intermediate to those found in patients with depression and those in healthy individuals without a family history of affective disorders [Mannie et al. 2009; Christensen et al. 2006]. We hypothesized that 4 weeks of treatment with escitalopram would improve cognitive function compared with placebo.
Methods and materials
The AGENDA (Associations between Gene-polymorphisms, Endophenotypes for Depression and Antidepressive Intervention) trial was designed as a participant, investigator, observer, and data-analyst-blinded randomized trial in which healthy first-degree relatives of patients with MDD received either escitalopram 10 mg/day or matching placebo for a period of 4 weeks. The trial was conducted from July 2007 until July 2009 at the Department of Psychiatry, Rigshospitalet, University Hospital of Copenhagen, Denmark, as part of the Center for Pharmacogenomics, University of Copenhagen. The trial was conducted and monitored in accordance with the International Conference on Harmonisation for Good Clinical Practice guidelines and the Declaration of Helsinki 2002. The trial protocol including sample size estimation was published before completion of the trial [Knorr et al. 2009]. Cognitive function was pre-defined as a secondary outcome measure of the AGENDA trial.
Assessments
The first part of the assessment was a telephone interview with the potential participants. The individuals eligible were scheduled to meet at the clinic both before and following 4 weeks of intervention. On the first day of examination the participants gave written informed consent after details of the trial were explained. Diagnoses were ascertained by the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) [Wing et al. 1990] and the structured Clinical Interview for DSM-IV Axis II Personality Disorders [First et al. 1997]. Further assessment included information on family history of psychiatric disorders, ratings of mood using the 17-item Hamilton Depression Rating Scale (HAM-D) [Bech et al. 1986], and 14-item Hamilton Anxiety Scale [Bech et al. 1986], various sociodemographics, height, weight, routine blood tests, and a pregnancy test for women. Participants self-rated depressive symptoms by Beck Depression Inventory, 42-items [Beck et al. 1961]. The neuropsychological test battery was applied on the same day as the interview and repeated following 4 weeks of intervention. The Side Effect Rating Scale by UKU-SERS-Pat [Lindstrom et al. 2001] was applied by the principal investigator (UK) to assess side effects following 4 weeks of intervention.
Randomization
Randomization to one of the two intervention groups was done on the first day of examination immediately after it had been established that a participant fulfilled all the inclusion criteria and none of the exclusion criteria (Figure 1). The Copenhagen Trial Unit (CTU) performed the centralized computerized randomization by telephone to secure adequate allocation sequence generation and allocation concealment. Randomization was stratified in blocks of six by age (18–31 years and 32–60 years) and sex. Only the data manager knew the block size. Participants were randomized 1:1 to receive either escitalopram 10 mg/day or placebo.
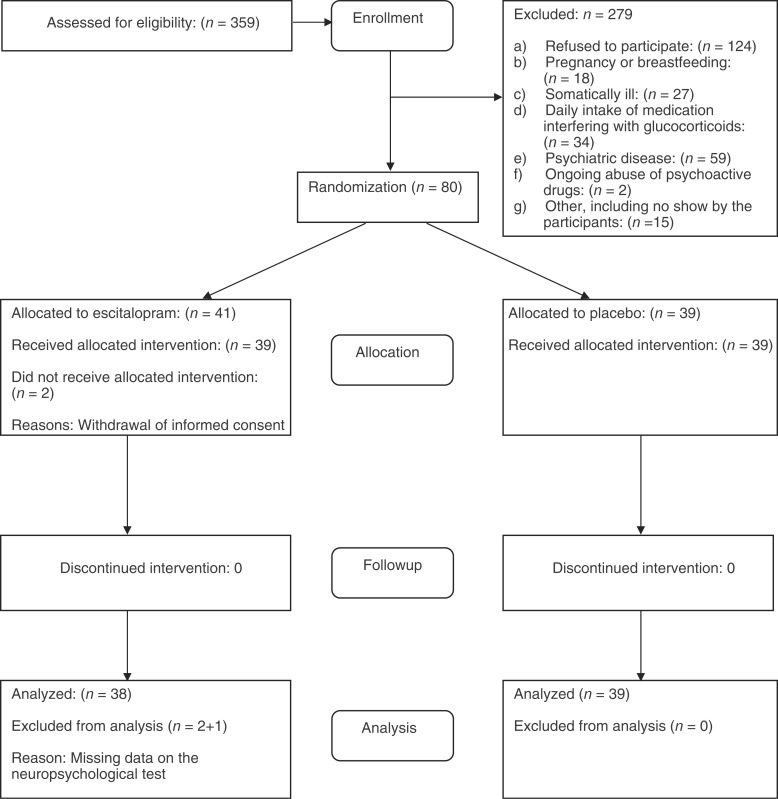
Figure 1.
Flowchart for the AGENDA Trial on Cognitive Function.
Blinding
All trial personnel and participants were blinded to the packaging of the trial drug, and blinding was maintained throughout monitoring, follow up, data management, assessment of outcomes, and data analyses. The randomization code was not broken until all data had been analysed and conclusions drawn, as suggested previously [Gotzsche, 1996]. At the assessment after 4 weeks of intervention, every participant and the principal investigator (UK) made a guess as to which intervention the participant had received. A large proportion of the participants said, ‘I do not know’ but were asked to give their best guess. The agreement between the actual intervention and the guesses was estimated to assess the degree to which blinding had been demasked, thus κ < 0, no; κ = 0.0–0.20, slight; κ = 0.21–0.40, some; κ = 0.41–0.60, moderate; κ = 0.61–0.80, substantial; κ = 0.81–1.00, almost complete demasking.
Interventions
The participants were randomized to self-administer a single dose of either escitalopram 10 mg or matching placebo each evening for 4 weeks. The rationale for evening administration of the intervention was to minimize possible discomfort by nausea. Escitalopram and placebo tablets were identical in appearance, colour, smell, and solubility allowing for blinding of the assignment to intervention or placebo. H. Lundbeck A/S provided identically appearing blister packages containing escitalopram or placebo. An independent pharmacist then packed, sealed, and numbered the drug packages according to a randomization list provided and concealed by the CTU. Adherence to the protocol was sought by making weekly telephone calls to the enrolled participants. The participants were asked at the end of the trial how adherent they had been to the protocol, and if they had missed taking any tablets. On completion of 4 weeks of intervention participants entered a 5-day blinded down-titration period to nil medication.
Neuropsychological tests
Cognitive functions were measured with neuropsychological tests at baseline and following 4 weeks of intervention. Descriptions of most of these tests may be found in ‘A compendium of neuropsychological tests’ [Strauss et al. 2006] and modifications are noted below. The 45-word Danish version of National Adult Reading Test (DART-45) [Nelson and O'Connell, 1978] was used as a measure of intelligence. Thirteen measures from the other tests were subjected to factor analysis, yielding the following four factors.
Factor 1. Visuomotor/visuospatial function
This factor included five measures: Trail Making A & B, connecting numbers (A) and alternating numbers and letters (B); Symbol Digit Modalities Test (SDMT), a sensitive test requiring the subject to write numbers corresponding to each of nine symbols indicated in a coding key, in 90 seconds; Block Design [Gade et al. 1988] a variant of the WAIS subtest with a score made up of the mean time in seconds to complete each of 12 designs with four blocks with red, white, and half red/white sides; Rey–Osterrieth Complex Figure, 3-minute free recall (copy score not included).
Factor 2. Executive function
This included four measures: two verbal fluency tests, phonological fluency (letter s) and semantic fluency (animals), each with number of words generated in 60 seconds; Letter–Number Sequencing is a working memory test also included in the WAIS-III, where the subject is read a combination of numbers and letters and is asked to reproduce the numbers first in ascending order and then the letters in alphabetic order; the Stroop Test, a measure of selective attention and cognitive control, requiring the subject to name the colour of ink of printed colour words, e.g. ‘blue’ printed in red. We used a version [Ravnkilde et al. 2002] previously used in depression and included in analyses only the time to name the colours in the incongruent part.
Factor 3. Verbal function
This included two tests, which may also be considered as tests of semantic memory: Familiar Faces [Waldemar et al. 1994] with naming of 28 generally well-known faces; and Boston Naming Test with 60 objects in line drawings.
Factor 4. Verbal learning and memory
This consisted of two measures from Rey Auditory Verbal Learning Test (RAVLT), which is a test of free recall of a list of 15 words. We included total number of words recalled in trials 1–5 and delayed recall after 30 minutes.
CAMCOG
In addition, UK examined all participants with the CAMCOG [Roth et al. 1986], the cognitive section of The Cambridge Examination for Mental Disorders of the Elderly (CAMDEX), which is a brief neuropsychological instrument that includes measures of language processing, working memory, and declarative memory. The maximum score was 104 points.
Analyses of neuropsychological test results
All scores of the cognitive tests (except CAMCOG) were transformed to Z-scores with a mean of 0 and an SD of 1 to allow grouping of highly correlated tests into factor scores. Factors scores were computed as the average of constituent test measures and standardized so all factors had a mean of 0 and an SD of 1. Similarly, the averages of all 13 tests measures were computed and standardized to create a global summary, here termed the ‘general cognition score’. The primary outcome measure of cognitive function was change in the general cognition score, calculated as the change in the general cognition score from trial entry to after 4 weeks of intervention (T4–T0). The general cognition score was constructed in order to have only one primary outcome measurement for cognitive function. Further, post hoc analyses were performed on each of the factors and on each of the individual tests.
To estimate reliabilities of test measures, we calculated test–retest correlations in all test measures (raw scores, factor scores and general cognition score) in the placebo group.
Test procedures
Three graduate psychology students trained and supervised by an experienced neuropsychologist (AG) conducted the neuropsychological testing. All tests were conducted in the same office, and all testing procedures were the same during the trial period. The same tester performed both the baseline and the follow-up test, which was performed at the same time during the day. The aim of the neuropsychological testing was explained to the participants and they were instructed the same way on both days of testing
Analysis of plasma escitalopram
Plasma escitalopram was measured following 4 weeks of intervention. The extraction and quantitation of escitalopram was carried out on an ASPEC XL combined with a high-pressure liquid chromatography (HPLC) system, both from Gilson, Villiers le Bell, France. Method validation resulted in lower and upper limits of quantitation of 10 and 3,600 nmol/l, respectively. The interassay coefficients of variation ranged from 5.5% to 8.4%, and trueness ranged from 93.2% to 103.0% within the measurement range. Extraction recovery was 38%, and carry-over was less than 1%.
Statistical methods
Data analyses were described in a pre-established analysis plan [Knorr et al. 2009]. All randomized participants were analysed, including those with missing data at the testing after 4 weeks of intervention. Statistical analyses were planned as analysis of covariance (ANCOVA) [Vickers and Altman, 2001] but if the mean of the change in the difference between the results for the general cognition score and factor scores before and after the intervention did not follow (and could not be transformed into) a normal distribution, the intervention groups were compared by a nonparametric test (Mann–Whitney U-test). Further, the outcomes were analysed as planned as the difference for the individual participants before and after the intervention, first unadjusted and then adjusted for age, sex, Hamilton depression score at entry, and the Danish Adult Reading Test, and concentration of escitalopram in plasma, if they presented with a p-value < 0.1 in the univariate analyses.
Results
Participant and non-participant characteristics
The probands (n = 466) gave us permission to contact 359 first-degree relatives, who were the potential participants in the trial. The participant flow, including reasons for exclusion, is shown in Figure 1. A total of 80 participants were included and randomized. The characteristics of the participants can be seen in Table 1.
Table 1.
Characteristics of the participants of the AGENDA trial at entry.
| Escitalopram (n = 41) | Placebo (n = 39) | All (n = 80) | |
|---|---|---|---|
| Age – years, mean ± SD | 32 ± 11 | 31 ± 11 | 32 ± 10 |
| Women – N (%) | 15 (37) | 14 (36) | 29 (36) |
| Proband was/– N (%) | |||
| Sibling | 18 (44) | 15 (39) | 33 (41) |
| Parent | 23 (56) | 24 (62) | 47 (59) |
| White – (%) | 100 | 100 | 100 |
| Education – mean ± SD | |||
| Years of school | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| Further education score | 3 ± 2 | 3 ± 2 | 3 ± 2 |
| Employment status – N (%) | |||
| Employed | 30 (73) | 26 (67) | 56 (70) |
| Student | 11 (27) | 11 (28) | 22 (28) |
| Unemployed | 0 (0) | 2 (5) | 2 (3) |
| First-degree relatives of patient with a history of major depressive disorder – median (quartiles)* | 1 (1;2) | 1 (1;2) | 1 (1;2) |
| Second-degree relatives with a history of major depressive disorder – median (quartiles) | 0 (0;1) | 0 (0;1) | 0 (0;1) |
| 17-item Hamilton Depression Scale Score, – median (quartiles) (range) | 1 (0;3) | 1 (0;3) | 1 (0;3) |
| (0–7) | (0–7) | (0–7) | |
| 14-item Hamilton Anxiety Scale Score, – median (quartiles) (range) | 1 (0;2) | 1 (0;2) | 1 (0;2) |
| (0–9) | (0–6) | (0–9) | |
| Beck Depression Inventory, 21-item, depression – median (quartiles) | 2 (0;4) | 2 (0;3) | 2 (0;5) |
| Beck Depression Inventory, 14-item, anxiety – median (quartiles) | 1 (0;4) | 2 (0;3) | 1 (0;3) |
| Daily medicine – N (%)** | 2 (5) | 4 (10) | 6 (8) |
| Danish Adult Reading Test 45 words mean ± SD*** (range) | 24.4 ± 8.4 | 25.1 ± 7.4 | 24.4 ± 7.8 |
| (6–40) | (8–38) | (6–40) | |
Notes: Two participants smoked cannabis more than 2 months prior to the investigation. Three were previously abusing alcohol. One participant had generalized anxiety.
Quartiles reported, are the 25 and 75 quartiles.
No benzodiazepines or antihistamines.
Missing data for one participant with dyslexia.
Adherence to the intervention
One or two tablets were missed by five participants in the placebo arm and by six participants in the escitalopram arm. In the escitalopram arm two participants left the trial prior to onset of the intervention period: one man withdrew the informed consent and one female started steroid treatment due to recurrence of skin allergy. Further, data is missing for one man for the follow-up test, except for CAMCOG, due to the participant's schedule problem. Full adherence to the protocol was stated by 32 participants in the placebo arm and by 33 in the escitalopram arm.
Plasma escitalopram
Blood was drawn from all 78 participants at follow up, but one test from the escitalopram group failed. The mean concentration of escitalopram was 50 nmol/l, SD 29 nmol/l, median 48 nmol/l, and range < 10 to 138 nmol/l (n = 38) in escitalopram group. Two participants from the escitalopram group had undetectable plasma escitalopram, thus < 10 nmol/l, one of which had stated missing the last two tablets prior to blood sampling. Plasma escitalopram was undetectable in all participants of the placebo group.
The neuropsychological tests
The test results at entry are presented in Table 2. The dataset for the neuropsychological tests was complete for 77 participants (96 %) both before (T0) and following 4 weeks of intervention (T4). Both groups improved considerably, presumably due to retest effects (positive values in Z-scores). The change in the general cognitive function score was normally distributed (Shapiro–Wilkes test). Accordingly we tested the difference between the two intervention arms with a t-test, but the difference was insignificant (p = 0.37, see Table 3).
Table 2.
Neuropsychological test results at baseline for 80 first-degree relatives of patients with major depressive disorder whom participated in the AGENDA trial.
| Neuropsychological test | Mean | Median | SD | 25 percentile | 75 percentile |
|---|---|---|---|---|---|
| Symbol Digit Modalities Test | 55 | 56 | 9 | 49 | 60 |
| Trail Making A | 28 | 27 | 9 | 21 | 35 |
| Trail Making B | 63 | 60 | 21 | 49 | 73 |
| Rey–Osterrieth Complex Figure, 3 min. | 22 | 23 | 7 | 19 | 27 |
| Block designs, seconds | 14 | 12 | 8 | 10 | 16 |
| Fluency for letter s | 17 | 17 | 5 | 13 | 19 |
| Fluency for animals | 26 | 26 | 6 | 23 | 29 |
| Letter number sequencing | 12 | 12 | 3 | 11 | 13 |
| Stroop (incongruence) | 107 | 102 | 24 | 91 | 122 |
| Familiar faces naming | 18 | 20 | 7 | 12 | 24 |
| Boston Naming | 56 | 57 | 3 | 53 | 58 |
| Rey Auditory Verbal Learning Test (A1A5) | 50 | 50 | 8 | 43 | 56 |
| Rey Auditory Verbal Learning Test (delay) | 11 | 10 | 3 | 8 | 13 |
| Cambridge Cognitive Examination | 97 | 97 | 3 | 96 | 99 |
Table 3.
The distribution of changes (Δ) in results of neuropsychological test measures, perceived stress and mood in first-degree relatives of the AGENDA trial following 4 weeks of intervention with escitalopram (n = 38) and placebo (n = 39).
| Quantity | Arm (n) | Mean (SD) | Median | Minimum value | Maximum value | Inter quartile range | p-value | Normality conditionsa |
|---|---|---|---|---|---|---|---|---|
| Δ General cognition score | Escitalopram | 1.17 | 1.28 | −0.23 | 2.23 | 0.89 | 0.37 | N |
| (0.55) | ||||||||
| Placebo | 1.04 | 1.06 | −0.26 | 2.35 | 0.97 | |||
| (0.69) | ||||||||
| Δ Factor 1 Visuomotor, visuospatial function | Escitalopram | 0.54 | 0.49 | −0.10 | 1.55 | 0.48 | 0.82 | − N |
| (0.39) | ||||||||
| Placebo | 0.42 | 0.45 | −0.64 | 1.95 | 0.93 | |||
| (0.58) | ||||||||
| Δ Factor 2 Executive function | Escitalopram | 0.39 | 0.45 | −0.64 | 1.95 | 0.93 | 0.27 | N |
| (0.58) | ||||||||
| Placebo | 0.23 | 0.11 | −0.93 | 1.75 | 0.84 | |||
| (0.64) | ||||||||
| Δ Factor 3 Verbal function | Escitalopram | 0.26 | 0.26 | −0.34 | 1.02 | 0.51 | 0.86 | N |
| (0.35) | ||||||||
| Placebo | 0.24 | 0.17 | −0.59 | 1.27 | 0.51 | |||
| (0.38) | ||||||||
| Δ Factor 4 Verbal learning and memory | Escitalopram | 0.95 | 0.95 | −0.61 | 2.54 | 0.90 | 0.41 | (N) |
| (0.66) | ||||||||
| Placebo | 1.05 | 1.16 | −0.79 | 3.38 | 0.72 | |||
| (0.78) | ||||||||
| Δ CAMCOG score | Escitalopram* | 1.21 | 1 | −5 | 5 | 2 | 0.04 | (N) |
| (1.92) | ||||||||
| Placebo | 2.16 | 2 | −2 | 6 | 3 | |||
| (1.98) | ||||||||
| Δ Hamilton score | Escitalopram | −0.2 | 0 | −4 | 3 | 2 | 0.88 | (N) |
| (1.5) | ||||||||
| Placebo | −0.1 | 0 | −5 | 4 | 2 | |||
| (1.7) | ||||||||
| Δ Beck Depression Inventory score | Escitalopram | −0.6 | 0 | −21 | 9 | 2 | 0.49 | −N |
| (4.6) | ||||||||
| Placebo | −1.9 | 0 | −14 | 5 | 6 | |||
| (4.8) |
Factor 1: Symbol Digit Modalities Test, Trail Making A and B, Rey–Osterrieth Complex Figure 3 min and Block designs.
Factor 2: Fluency for letter s, Fluency for animals, Letter number sequencing, Stroop (incongruence).
Factor 3: Familiar faces naming and Boston Naming
Factor 4: Rey Auditory Verbal Learning Test A1A5 and delay.
Δ: The difference (T4–T0) between the measurement after (T4) and before (T0) 4 weeks of intervention with escitalopram 10 mg or placebo.
The symbols used in this column are to be interpreted as follows N: the distributions did not differ significantly from the normal distribution (Shapiro–Wilkes test); (N): they did differ but judged from the graphical displays (histograms and probability distributions) they followed normal distributions with reasonable approximation; –N: they did not follow normal distributions. In the first case a Student's t-test was applied. In the last two cases the distributions were compared using a Mann–Whitney U-test.
n = 39 in the escitalopram group for CAMCOG score.
In univariate analyses no statistically significant correlations were found between change in the general cognitive function score and age, sex, Hamilton depression score at entry, Danish Adult Reading Test, and plasma escitalopram. In post hoc explorative analyses of the factors 1–4 individually, and of the individual tests, no statistically significant differences were found between the escitalopram group and the placebo group. For the CAMCOG test, there was a statistically significant difference between the intervention groups, however, in contrast to the hypothesis, treatment with escitalopram improved the CAMCOG score less than placebo (1.21 [SD 1.92] versus 2.16 [SD 1.98], p = 0.04, Table 3).
Mood
In analyses of mood, no statistically significant differences were found between the escitalopram group and the placebo group, Table 3.
Discussion
Our hypothesis that an intervention with escitalopram 10 mg would have specific beneficial effects on cognitive function in healthy first-degree relatives of patients with MDD was not supported. Thus, there was no statistically significant difference between the change in cognitive function following 4 weeks of intervention with escitalopram 10 mg/day compared with matching placebo in healthy first-degree relatives of patients with MDD. Further, no statistically significant differences were seen in change in scale scores of mood between the two intervention groups. The finding in the CAMCOG test is most likely a type 1 error since many outcomes were explored in this trial. Taking multiple testing into account and correcting for that would also make this finding insignificant. Consequently, improvement in cognitive function after treatment with SSRI in depressed patients seems to be related to the effects on depression symptoms.
Advantages of the AGENDA trial
The present trial is distinguished by fulfilling the criteria in the Consort Statement guidelines, and by including healthy individuals with a family history of depression only. In contrast to most studies [Knorr and Kessing, 2010] we present information on factors that may influence outcomes such as age, gender, drug levels, depression score and ethnicity. It is an advantage that the trial and the analyses were carried out as planned and the completion in the trial was very high. No participants dropped out due to adverse events. The participants were studied in a randomized clinical trial blinded in all phases including the statistical analyses and conclusion phase. The blinding was successful in relation to participants as well as researchers. Furthermore, the registered diagnoses of depression for the probands were verified by a face-to-face psychiatric research interviews by trained medical doctors. The participants were assessed and diagnosed by validated and frequently used multidimensional methods (e.g. SCAN interviews). In addition, the participants were genetically homogeneous as all were ethnic Danes with European, mostly Danish, parents and grandparents. We used well-established methods for evaluations of cognitive function and we increased reliability and thus sensitivity by combining test measures. Finally, the antidepressant effect of escitalopram is generally accepted [Knorr and Kessing, 2010; Cipriani et al. 2009; Turner et al. 2008] and the participants were subjected to 4 weeks of intervention thus including the interval in which clinical improvement has been reported in trials on patients with MDD.
Limitations of the trial
It is a limitation that we have not compared healthy individuals with and without a family history of MDD. Thus, it would be a stronger conclusion had the participants been shown to have cognitive deficits. Further, a large number of women were excluded from our trial due to oral contraceptives and pregnancy, thus the trial population is characterized by an overrepresentation of men. We cannot exclude that the dosage of 10 mg escitalopram was too low although this has been suggested as the optimum dose for treatment of moderate depression [Bech et al. 2006]. Even though the participants received weekly phone calls to optimize adherence, some of the participants in the escitalopram group were found to have low plasma escitalopram concentrations. Our serum escitalopram concentrations were lower than in a study by Sogaard and colleagues [Sogaard et al. 2005], who found steady-state plasma escitalopram concentrations of 63 ± 32 nmol/l for escitalopram 10 mg as compared with 50 ± 29 nmol/l in our trial. The low plasma levels in our trial may be a result of the fact that approximately 12 hours elapsed from taking the last tablet to blood sampling.
We have considered using a higher dosage, but escitalopram 20 mg daily might have given more adverse effects, possibly jeopardizing blinding and adherence. The dose of escitalopram 10 mg used resulted in well-known adverse effects as described in previous papers [Knorr et al. 2011; Wingen et al. 2005].
Risk of errors
We have minimized the risk of systematic error (‘bias’) by using a randomized, age- and sex-stratified sample, and comparison with blinding in all phases of the trial. Also our neutral results speak against any bias. We planned to include 80 participants due to resources, feasibility and availability of the healthy first-degree relatives of patients with MDD. The AGENDA trial was planned and executed as a superiority trial and was not designed as an equivalence or noninferiority trial [Christensen, 2007]. Hence, we cannot exclude the possibility of overlooking a difference due to random error (‘play of chance’). This issue can only be solved by further trials [Sogaard et al. 2005]. Finally, we have analysed multiple outcomes thus increasing the risk of type I error for the remaining outcomes of the trial, as previously described [Knorr et al. 2009].
Generalizability
To increase the chances of detecting an effect of escitalopram versus placebo we included healthy individuals at increased risk of developing depression (i.e. with a first-degree family history of depression), as these participants seem to be to present with subtle cognitive dysfunction as previously shown in a study from our group [Christensen et al. 2006]. Further, as no effect of escitalopram was found in the present trial including a group of participants at enhanced risk this finding may be generalized to healthy Whites without a family history of depression.
Conclusion
Our results suggest that treatment with escitalopram does not improve or impair cognitive function in healthy individuals with a first-degree family history of severe depression. Improvement in cognitive function following treatment of depressed patients with SSRIs seems to be related to the effects on depressive symptoms rather than to a direct effect of the SSRI.
Trial registration
Local Ethics Committee: H-KF 307413.
Danish Medicines Agency: 2612-3162.
EudraCT: 2006-001750-28.
Danish Data Agency: 2006-41-6737.
ClinicalTrials.gov identifier: NCT 00386841 (AGENDA).
Acknowledgements
The members of the data monitoring and safety committee, Associate Professor Jørgen Hilden and Professor Per Bech, are thanked for their contribution. Vibe Nordahn Bredsdorff, Helene Dysgaard and Peter Kristian Jacobsen conducted the neuropsychological tests. We thank H. Lundbeck A/S for the free supply of the trial drug and placebo, and the Eli Larsen Foundation, the Jeppe Juhl Foundation, the Geert Jørgensen Foundation and the Ivan Nielsen Foundation for unrestricted economical support. Research secretary Helen Gerdrup Nielsen and Good Clinical Practice coordinator Kristian Juul are thanked for their valuable contribution to the trial.
Footnotes
This work was supported by The Danish Research Council, University of Copenhagen and the Lundbeck Foundation. Ulla Knorr was supported by a fellowship from the Center for Pharmacogenomics, University of Copenhagen.
The trial was fully investigator initiated and controlled to secure unbiased assessment of the effect of escitalopram on healthy first-degree relatives of patients with depression. AG, PW, CG, JW and UG declare to have no competing interests. UK has been a speaker for Servier. MV has been a speaker for Eli Lilly, Wyeth, Jannsen-Cilag, AstraZeneca and Pfizer. LVK has been a consultant for Bristol-Myers Squibb, Eli Lilly, Lundbeck, AstraZeneca, Servier and Jannsen-Cilag. The AGENDA trial has received nonrestricted grants from not-for-profit and for-profit organizations.
References
- Allen D, Lader M, Curran HV. (1988) A comparative study of the interactions of alcohol with amitriptyline, fluoxetine and placebo in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry 12: 63–80 [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. (2001) Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178: 200–206 [DOI] [PubMed] [Google Scholar]
- Bech P, Andersen HF, Wade A. (2006) Effective dose of escitalopram in moderate versus severe DSM-IV major depression. Pharmacopsychiatry 39: 128–134 [DOI] [PubMed] [Google Scholar]
- Bech P, Kastrup M, Rafaelsen OJ. (1986) Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl 326: 1–37 [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. (1961) An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571 [DOI] [PubMed] [Google Scholar]
- Christensen E. (2007) Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 46: 947–954 [DOI] [PubMed] [Google Scholar]
- Christensen MV, Kyvik KO, Kessing LV. (2006) Cognitive function in unaffected twins discordant for affective disorder. Psychol Med 36: 1119–1129 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. (2009) Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373: 746–758 [DOI] [PubMed] [Google Scholar]
- Drueke B, Baetz J, Boecker M, Moeller O, Hiemke C, Grunder G, et al. (2009) Differential effects of escitalopram on attention: a placebo-controlled, double-blind cross-over study. Psychopharmacology (Berl) 207: 213–223 [DOI] [PubMed] [Google Scholar]
- Fairweather D, Pozzo C, Kerr J, Lafferty S, Hindmarch I. (1997) Citalopram compared to dothiepin and placebo: effects on cognitive function and psychomotor performance. Human Psychopharmacol 12: 119–126 [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. (1997) The Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), American Psychiatric Press: Washington, DC [Google Scholar]
- Gade A, Mortensen EL, Bruhn P. (1988) ‘Chronic painter’s syndrome’. A reanalysis of psychological test data in a group of diagnosed cases, based on comparisons with matched controls. Acta Neurol Scand 77: 293–306 [DOI] [PubMed] [Google Scholar]
- Gotzsche PC. (1996) Blinding during data analysis and writing of manuscripts. Control Clin Trials 17: 285–290 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. (2009a) Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 195: 102–108 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. (2009b) Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 166: 1178–1184 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. (2004) Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry 161: 1256–1263 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV. (2010) Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. J Affect Disord http://www.ncbi.nlm.nih.gov.ep.fjernadgang.kb.dk/pubmed?term=hasselbalch%20and%20knorr. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kessing LV. (1998) Cognitive impairment in the euthymic phase of affective disorder. Psychol Med 28: 1027–1038 [DOI] [PubMed] [Google Scholar]
- Knorr U, Kessing LV. (2010) The effect of selective serotonin reuptake inhibitors in healthy subjects. A systematic review. Nord J Psychiatry 64: 153–163 [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Hansen A, Klose M, Feldt-Rasmussen U, Hasselstrom J, et al. (2011) Escitalopram and neuroendocrine response in healthy first-degree relatives to depressed patients; a randomized blinded trial. Unpublished Work . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Klose M, Feldt-Rasmussen U, Hilsted L, Gade A, et al. (2009) Rationale and design of the participant, investigator, observer, and data-analyst-blinded randomized AGENDA trial on associations between gene-polymorphisms, endophenotypes for depression and antidepressive intervention: the effect of escitalopram versus placebo on the combined dexamethasone-corticotrophine releasing hormone test and other potential endophenotypes in healthy first-degree relatives of persons with depression. Trials 10: 66–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cole S, Wolkowitz O, Reus V, Chan T, Moore E. (1997) Serotonergic intervention increases affiliative behavior in humans. Ann N Y Acad Sci 807: 492–493 [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, et al. (1998) Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 155: 373–379 [DOI] [PubMed] [Google Scholar]
- Lindstrom E, Lewander T, Malm U, Malt UF, Lublin H, Ahlfors UG. (2001) Patient-rated versus clinician-rated side effects of drug treatment in schizophrenia. Clinical validation of a self-rating version of the UKU Side Effect Rating Scale (UKU-SERS-Pat). Nord J Psychiatry 55(Suppl. 44): 5–69 [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Tombari D, Pariente J, Gerdelat-Mas A, Franceries X, Cassol E, et al. (2005) Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. Neuroimage 27: 299–313 [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Barnes J, Bristow GC, Harmer CJ, Cowen PJ. (2009) Memory impairment in young women at increased risk of depression: influence of cortisol and 5-HTT genotype. Psychol Med 39: 757–762 [DOI] [PubMed] [Google Scholar]
- Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ. (2007) Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage 37: 904–911 [DOI] [PubMed] [Google Scholar]
- Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ. (2008) Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int J Neuropsychopharmacol 12: 169–179 [DOI] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. (1978) Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 14: 234–244 [DOI] [PubMed] [Google Scholar]
- Paul MA, Gray G, Lange M. (2002) The impact of sertraline on psychomotor performance. Aviat Space Environ Med 73: 964–970 [PubMed] [Google Scholar]
- Paul MA, Gray GW, Love RJ, Lange M. (2007) SSRI effects on pyschomotor performance: assessment of citalopram and escitalopram on normal subjects. Aviat Space Environ Med 78: 693–697 [PubMed] [Google Scholar]
- Peran P, Demonet JF, Cardebat D. (2008) Paroxetine-induced modulation of cortical activity supporting language representations of action. Psychopharmacology (Berl) 195: 487–496 [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Muntjewerff ND, O'Hanlon JF. (1995) A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br J Clin Pharmacol 39: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. (2002) Cognitive deficits in major depression. Scand J Psychol 43: 239–251 [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Eikmans K, Heldens A, Schmitt JA. (2005) Specific serotonergic reuptake inhibition impairs vigilance performance acutely and after subchronic treatment. J Psychopharmacol 19: 12–20 [DOI] [PubMed] [Google Scholar]
- Robbe HW, O'Hanlon JF. (1995) Acute and subchronic effects of paroxetine 20 and 40 mg on actual driving, psychomotor performance and subjective assessments in healthy volunteers. Eur Neuropsychopharmacol 5: 35–42 [DOI] [PubMed] [Google Scholar]
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. (1986) CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 149: 698–709 [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Kruizinga MJ, Riedel WJ. (2001) Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J Psychopharmacol 15: 173–179 [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Ramaekers JG, Kruizinga MJ, van Boxtel MP, Vuurman EF, Riedel WJ. (2002a) Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. J Psychopharmacol 16: 207–214 [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Riedel WJ, Vuurman EF, Kruizinga M, Ramaekers JG. (2002b) Modulation of the critical flicker fusion effects of serotonin reuptake inhibitors by concomitant pupillary changes. Psychopharmacology (Berl) 160: 381–386 [DOI] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. (2003) Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 168: 293–298 [DOI] [PubMed] [Google Scholar]
- Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. (2009) Major depression, cognitive dysfunction and Alzheimer's disease: Is there a link? Eur J Pharmacol 626: 72–82 [DOI] [PubMed] [Google Scholar]
- Sogaard B, Mengel H, Rao N, Larsen F. (2005) The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol 45: 1400–1406 [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. (2006) A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, Oxford University Press: New York [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358: 252–260 [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. (2001) Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 323: 1123–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemar G, Bruhn P, Kristensen M, Johnsen A, Paulson OB, Lassen NA. (1994) Heterogeneity of neocortical cerebral blood flow deficits in dementia of the Alzheimer type: a [99mTc]-d,l-HMPAO SPECT study. J Neurol Neurosurg Psychiatry 57: 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Bailey JE, Alford C, Weinstein A, Nutt DJ. (2002) Effects of 5 weeks of administration of fluoxetine and dothiepin in normal volunteers on sleep, daytime sedation, psychomotor performance and mood. J Psychopharmacol 16: 321–331 [DOI] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. (1990) SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 47: 589–593 [DOI] [PubMed] [Google Scholar]
- Wingen M, Bothmer J, Langer S, Ramaekers JG. (2005) Actual driving performance and psychomotor function in healthy subjects after acute and subchronic treatment with escitalopram, mirtazapine, and placebo: a crossover trial. J Clin Psychiatry 66: 436–443 [DOI] [PubMed] [Google Scholar]
- Wingen M, Langer S, Ramaekers JG. (2006) Verbal memory performance during subchronic challenge with a selective serotonergic and a mixed action antidepressant. Hum Psychopharmacol 21: 473–479 [DOI] [PubMed] [Google Scholar]