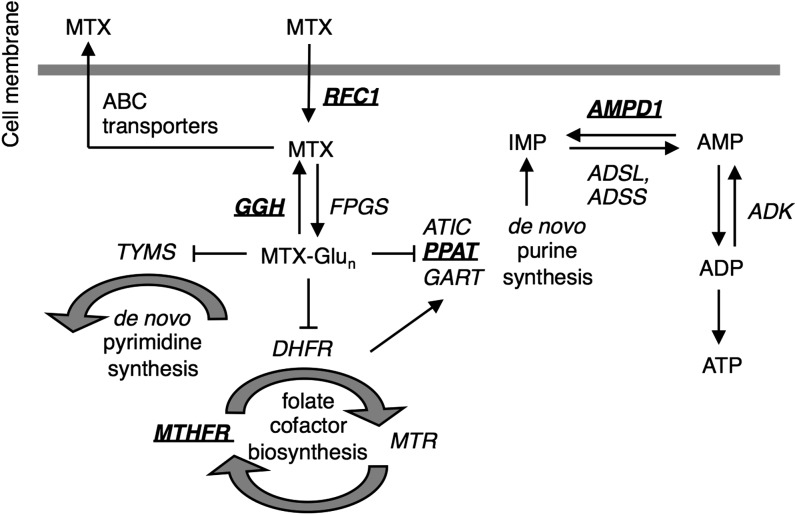
Figure 2.
MTX cellular pathway. MTX enters cancer cells via the reduced folate carrier, and the efflux across the cell membrane is mediated by various ABC transporters. Inside the cell, MTX is converted to active methotrexate polyglutamates by folylpolyglutamate synthetase (FPGS), which adds glutamate residues to MTX. The primary action of MTX is inhibition of the enzyme dihydrofolate reductase (DHFR), which converts dihydrofolate to tetrahydrofolate. The effect of MTX depends on the function and expression of several other enzymes in the folate pathway, including methylenetetrahydrofolate dehydrogenase, 5,10-methylenetetrahydrofolate reductase, and thymidylate synthetase. Degradation of MTXPGs to MTX depends on the activity of the lysosomal enzyme GGH, which catalyzes the removal of polyglutamates (Mikkelsen et al. 2011). AMP deaminase (AMPD1) converts adenosine monophosphate (AMP) to inosine monophosphate (IMP). Adenylosuccinate synthase (ADSS) and adenylosuccinate lyase (ADSL) counteract the action of AMPD1, convert IMP back to AMP in a two-step process. Adenylate kinase catalyzes the formation of two molecules of ADP from AMP and ATP; ADP is the substrate for oxidative phosphorylation that forms ATP. GSTs are not known to directly interact with methotrexate but are main mediators of detoxification by conjugation of xenobiotics with glutathione.