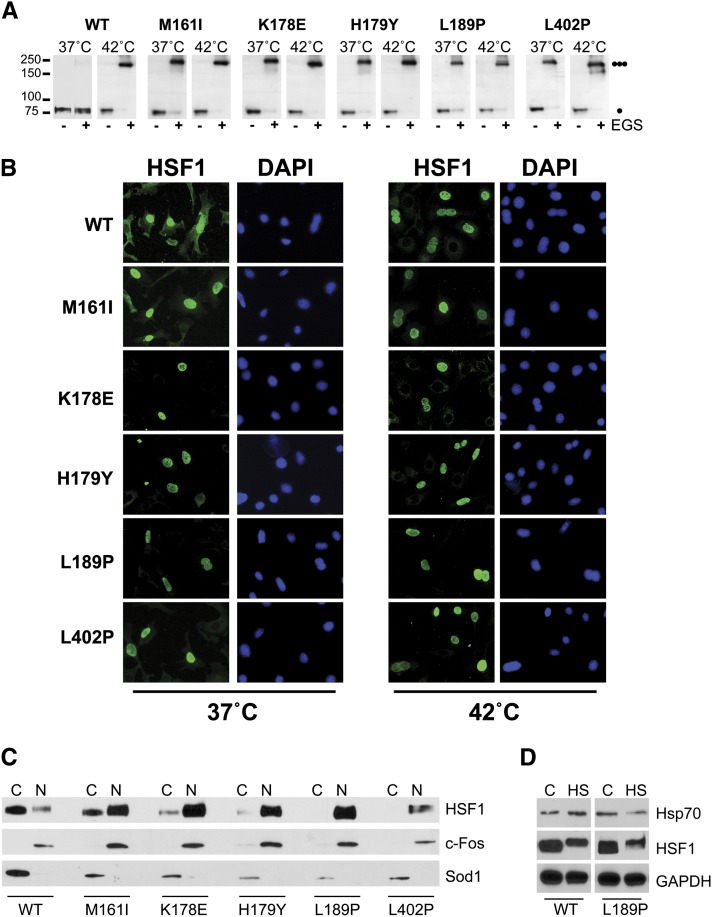
Figure 6.
HSF1 leucine zipper mutations promote constitutive trimerization and nuclear localization in mammalian cells. (A) hsf1−/− MEFs were transfected with a plasmid expressing wild-type HSF1 or the HSF1 mutants identified in this screen and analyzed for HSF1 multimerization by EGS cross-linking. The positions of molecular weight markers are indicated on the left, and circles indicating the expected migration of HSF1 monomers and trimers are on the right. (B) hsf1−/− MEFs were transfected with a plasmid expressing wild-type HSF1 or the HSF1 mutants identified in this screen and assayed for localization by immunofluorescence. (C) hsf1−/− MEFs were transfected with a plasmid expressing wild-type HSF1 or the HSF1 mutants identified in this screen and nuclear and cytoplasmic fractions were analyzed for HSF1 by immunoblotting. c-fos and SOD1 serve as nuclear (N) and cytoplasmic (C) markers, respectively. (D) hsf1−/− MEFs were transfected with a plasmid expressing wild-type HSF1 or the L189P HSF1 mutant, heat shocked at 42° for 30 min and protein extracts were assayed for HSF1 hyper-phosphorylation by immunoblotting for an HSF1 mobility shift. Total protein extracts were also immunoblotted for Hsp70 and GAPDH serves as a loading control.