Abstract
Exogenous ribonucleases are known to inhibit tumor growth via apoptosis induction in tumor cells, allowing to consider them as promising anticancer drugs for clinical application. In this work the antitumor potential of binase was evaluated in vivo and the mechanism of cytotoxic effect of binase on tumor cells was comprehensively studied in vitro. We investigated tumoricidal activity of binase using three murine tumor models of Lewis lung carcinoma (LLC), lymphosarcoma RLS40 and melanoma B-16. We show for the first time that intraperitoneal injection of binase at a dose range 0.1–5 mg/kg results in retardation of primary tumor growth up to 45% in LLC and RLS40 and inhibits metastasis up to 50% in LLC and RLS40 and up to 70% in B-16 melanoma. Binase does not exhibit overall toxic effect and displays a general systemic and immunomodulatory effects. Treatment of RLS40-bearing animals with binase together with polychemotherapy revealed that binase decreases the hepatotoxicity of polychemotherapy while maintaining its antitumor effect. It was demonstrated that the cytotoxic effect of binase is realized via the induction of the intrinsic and extrinsic apoptotic pathways. Activation of intrinsic apoptotic pathway is manifested by a drop of mitochondrial potential, increase in calcium concentration and inhibition of respiratory activity. Subsequent synthesis of TNF-α in the cells under the action of binase triggers extrinsic apoptotic pathway through the binding of TNF with cell-death receptors and activation of caspase 8. Thus binase is a potential anticancer therapeutics inducing apoptosis in cancer cells.
Keywords: RNase, cytotoxicity, apoptotic pathways, murine tumor model, tumoricidal activity
Introduction
Extensive studies of the antitumor potential of exogenous ribonucleases (RNases) derived from fungi, bacteria, plants and animals allow us to consider them as perspective therapeutics for treatment of oncological diseases.1-5 The antitumor activity was shown for BS-RNase from bovine seminal,6-9 onconase from oocytes of Rana pipiens,10-12 bovine pancreatic RNase A,13 cSBL and jSBL RNases from Rana catesbeiana and Rana japonica,14 microbial RNases15-17 and conjugates of RNases with various molecules.18-20
One of the properties that are important for potential therapeutic application of RNases is their ability to induce cancer cell death by apoptosis. However, despite a large number of studies of the cytotoxic effect of RNases on cells, key elements of the signaling network and the mechanism of apoptosis induction are poorly understood. The available data are fragmentary and insufficient to develop a strategy of anticancer therapy with RNases.
Molecular mechanisms underlying cytotoxicity of RNases are diverse. The main mechanism confirmed specifically for onconase lies in the cleavage of intracellular RNA triggering apoptosis or, presumably generating new set of small regulatory RNA molecules that affect gene expression in tumor cells.21
RNase from Bacillus intermedius (binase) is a long established effective agent for inhibition of tumor cell growth: it displays cytotoxic effects on human leukemic K562 and Kasumi-1 cells.22,23 It was shown that sensitivity of cells to binase toxic action depends on the expression of KIT, AML1-ETO and FLT3 oncogenes.23,24 At the same time, RNase cytotoxicity does not correlate with reduction of total amount of RNA.17,25 The possible mechanisms of cytotoxic effect of binase are as follows: cleavage of certain intracellular RNAs with generation of a novel set of small regulatory RNAs (miRNAs), startup of intrinsic apoptotic pathway through the changes in mitochondria, startup of extrinsic apoptotic pathway through the increase in the expression of some pro-apoptotic genes including cell death ligand TNF-α and activation of initiator caspase 8.26 However despite the fact that binase cytotoxicity toward tumor cells in vitro was well studied the investigation of tumoricidal activity of binase in vivo has not yet been performed.
In the present study, the ability of binase to retard primary tumor and metastasis growth was demonstrated for the first time on the three tumor models differing from each other by histological type and having strong relevance to human tumors: Lewis lung carcinoma, lymphosarcoma RLS40 and melanoma B-16. LLC and RLS40 are tumor models with the primary tumor site and metastasize in the lungs and liver, respectively; melanoma B-16 is a metastatic model with pulmonary metastases. The search for possible mechanism of antitumor activity of binase was done in vitro using flow cytometry and relevant lines of tumor cells.
Results
As a first step binase toxicity was evaluated on healthy C57Bl/6 and CBA mice treated with binase at doses of 1 and 5 mg/kg by intraperitoneal administration. Control groups included intact mice and mice with intraperitoneal administration of saline buffer. In assessing the binase toxicity, no differences between control mice and mice treated with binase for both C57Bl/6 and CBA lines were revealed. During the experiment, the body weight of mice, condition of the hair and skin, the motor (physical) activity, the consumption of food and water did not change, irrespective of binase dosage. The volume densities of the normal hepatocytes and destructive changes in liver tissue of intact mice and mice receiving saline buffer were 82.5 ± 1.5% and 10.5 ± 0.7% of the entire liver parenchyma, respectively. Administration of binase at a dose of 1 mg/kg caused no additional destructive changes in liver parenchyma. Administration of binase at a dose of 5 mg/kg led to statistically insignificant increase of the destructive changes in liver parenchyma of healthy mice. The numerical density of binuclear hepatocytes in liver of mice receiving binase did not differ from that of the control groups (1.7 ± 0.2 per unit of test area of histological section) in the whole dosage range, thus indicating absence of the reduction of regenerative capacity of liver tissue during the period of treatment.
The antitumor activity of binase has been evaluated in vivo applying criteria such as tumor size, number and area of metastases in the target organ, the regenerative potential of liver and the profile of cytokines in blood plasma.
Effect of binase on LLC tumor growth and metastasis development
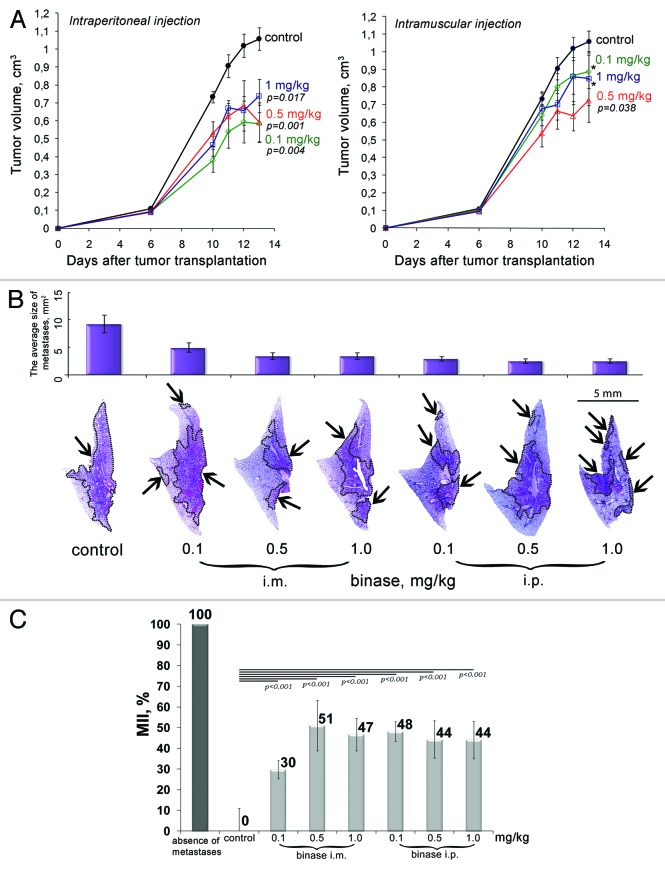
To assess the therapeutic potential of binase, the highly metastatic primary tumor of LLC was initiated by intramuscular injection of tumor cells into the right thighs of mice. To determine the most effective dosage and optimal mode of administration, binase was injected in two ways; intraperitoneally or intramuscularly at doses of 0.1, 0.5 and 1 mg/kg. The study of the dynamics of tumor growth revealed that over the entire dosage range intraperitoneal administration of binase led to a significant retardation of tumor growth, reaching 45% (Fig. 1A). After intramuscular administration of binase a tendency to a decrease in tumor volume was also observed; however, these differences were not statistically significant except for the dose of 0.5 mg/kg when tumor size was reduced by 35% (Fig. 1A). Evidently, intraperitoneal administration of binase has the highest therapeutic efficacy, which is probably due to better absorption of the enzyme in comparison with intramuscular administration.
Figure 1. Inhibition of primary tumor growth and metastases in mice with Lewis lung carcinoma under the treatment with binase. (A) The effect of binase on the growth rate of primary tumor. Binase was injected intraperitoneally (i.p.) or intramuscularly (i.m.) thrice a week at the doses of 0.1, 0.5 and 1 mg/kg starting on day 4 after tumor transplantation. (B) Representative histotopograms of lung lobes of mice treated with binase. Haematoxylin and eosin staining. Metastases are indicated by arrows, borders of metastases are outlined by dotted line. (C) Metastasis inhibition index (MII) in the control and experimental groups. MII = [mean metastasis areacontrol − mean metastasis areaexperiment]/mean metastasisareacontrol × 100%. p value indicates a statistically reliable difference relative to the control group, and (*) denotes no statistically significant difference.
Histological analysis revealed a distinct reduction in the area of lung metastasis in the groups of mice treated with binase (Fig. 1B). Inhibition of metastases development was assessed by morphometry using the metastasis inhibition index (MII). The MII of the control group was taken as 0% and the MII, corresponding to 100%, reflected the absence of metastases. Morphometric analysis showed that in the groups of mice with LLC that received intramuscular injections of binase at doses of 0.1, 0.5 and 1 mg/kg, the MII increases with the increase in a dose and was 30, 51 and 47%, respectively (Fig. 1C). In all groups of animals treated with intraperitoneal injections of binase, the MII was the average 50% (Fig. 1C). The obtained data suggest that intraperitoneal administration of binase is more potent than intramuscular treatment.
To evaluate the immunomodulatory effect of the binase therapy the levels of interferons promoting antitumor response and the levels of pro-inflammatory cytokines IL-1α, IL-6 and TNF-α in the blood serum of LLC-bearing mice were measured after the treatment with binase (data not shown). Intramuscular administration of binase resulted in a gradual increase in IFN-γ level from 157 ± 13 pg/ml in the blood of LLC-bearing mice to 540 ± 166 pg/ml in group of animals, intramuscularly treated with binase at the dose of 1 mg/kg. Intraperitoneal injections of binase also caused an increase of IFN-γ level, but to a lesser extent (up to 302 ± 79 pg/ml). It should be noted that although the increase in IFN-γ level differs insignificantly from the control, it has a dose-dependent behavior. Statistically significant increase of pleotropic cytokines IL-1α was observed only for intraperitoneally injected binase at the dose of 1 mg/kg. In these dosage ranges, binase had no effect on the levels of INF-α (< 5 pg/ml) and TNF-α (< 20 pg/ml).
Effect of binase on RLS40 tumor growth and metastasis development
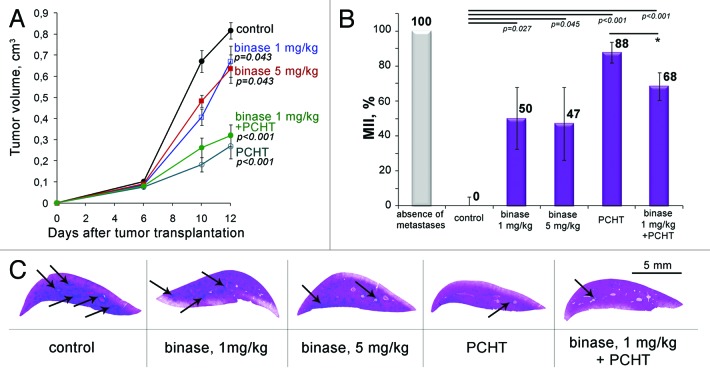
To elucidate the binase potential against tumors of various histological types, lymphosarcoma RLS40 exhibiting MDR phenotype,27 which forms the primary tumor site and is capable of metastasizing the liver, was further studied. One of the important objectives of this experiment was to assess whether the observed therapeutic effect of binase can be enhanced with the increase in a dosage of the enzyme up to 5 mg/kg. Additionally, we compared the antitumor effect of binase and conventional polychemotherapy (CHOP) and evaluated the potential of binase as, an adjuvant, in combination with chemotherapy. The study of dynamics of tumor growth showed that on day 10 after tumor initiation, in the groups of mice treated thrice with binase at doses of 1 and 5 mg/kg, retardation of tumor growth was 40 and 28%, respectively (Fig. 2A). At the end of the experiment, inhibition of tumor growth in these groups was near 20%. Thus the increase in the dose of binase to 5 mg/kg did not lead to the enhancement of antitumor effect. The treatment of mice with CHOP was 1.5–2-fold more efficient than monotherapy with binase and resulted in a 60–70% inhibition of tumor growth. The combined therapy with binase and CHOP did not lead to a potentiation of antitumor response; the retardation of tumor growth in this group was in line with the effect of CHOP monotherapy.
Figure 2. Inhibition of primary tumor growth and metastases in mice with lymphosarcoma RLS40 under the treatment with binase, polychemotherapy (PCHT) and combination of binase and PCHT. (A) The effect of binase, PCHT (CHOP regimen) and the combination of binase and PCHT on the growth rate of lymphosarcoma RLS40. Binase was injected intraperitoneally thrice a week at the doses of 1 and 5 mg/kg starting on day 4 after tumor transplantation, PCHT was injected twice on day 4 and 11 after tumor transplantation. (B) Metastasis inhibition index (MII) in the control and experimental groups. MII = [mean metastasisareacontrol − mean metastasis areaexperiment]/mean metastasisareacontrol × 100%. p value indicates a statistically reliable difference relative to the control group and (*) denotes no statistically significant difference. (C) Representative histotopograms of the liver lobes of mice treated with binase. Haematoxylin and eosin staining. Large metastases are indicated by arrows.
Histological and morphometric analysis revealed that the MII in the group of mice treated with CHOP slightly fluctuated within the groups and was 88% on average (Fig. 2B and C). The treatment of mice with binase resulted in 50% suppression of development of metastases (Fig. 2B and C). It should be noted that about half of the mice in binase-treated groups responded to the treatment, and no metastases were found. The combination of CHOP treatment and binase showed an intermediate effect; MII was 68%.
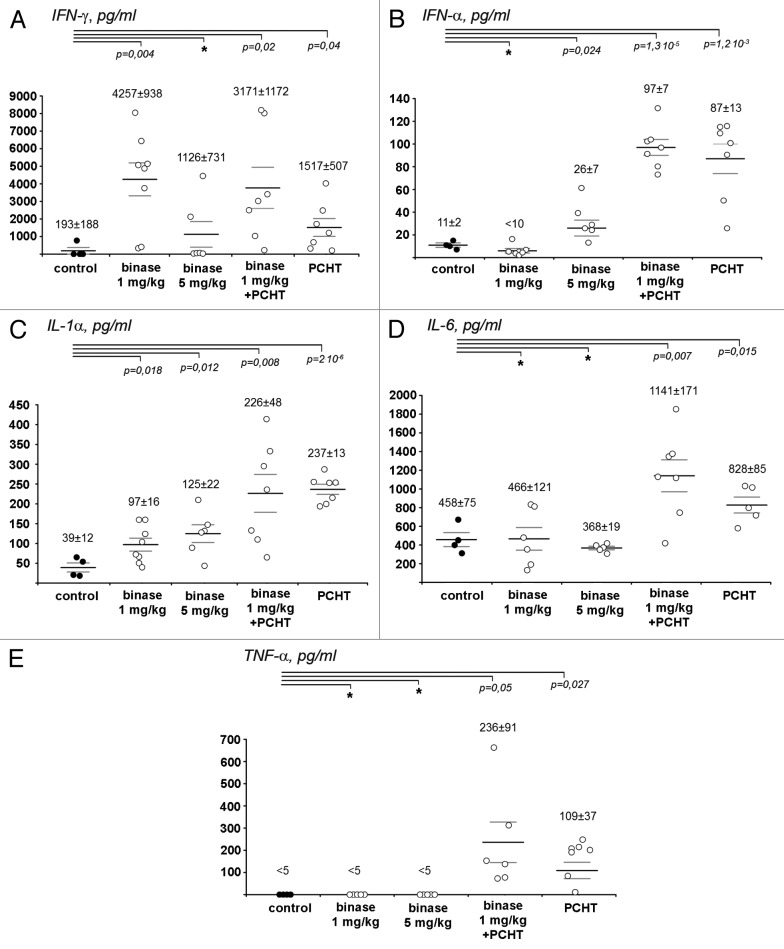
Binase at the dose of 1 mg/kg caused 20-fold increase of IFN-γ level in blood serum of RLS40-bearing mice in comparison with the group of animals without treatment (p = 0.004, Fig. 3A). CHOP monotherapy also resulted in 8-fold increase of IFN-γ level (p = 0.04). In the group of animals treated with CHOP and binase at the dose of 1 mg/kg, the IFN-γ level was comparable with the level in the serum of animals treated only with binase. Similar and statistically significant increase of IFN-α level was observed in the serum of mice treated both with the CHOP monotherapy and with combined therapy (Fig. 4B). Since binase itself at the dose of 1 mg/kg did not cause increase of IFN-α level, it is obvious that such increase in these groups was due to the effect of CHOP.
Figure 3. The change in cytokine profiles in the blood serum of RLS40-bearing mice after the treatment with binase, polychemotherapy (PCHT) and combination of binase and PCHT. (A) IFN-γ, (B) IFN-α, (C) IL-1α, (D) IL-6, (E) TNF-α. The levels of cytokines in the blood serum were measured by ELISA. For each group of animals the value of MEAN ± SEM is indicated. p value indicates a statistically reliable difference relative to the control group, and (*) denotes no statistically significant difference.
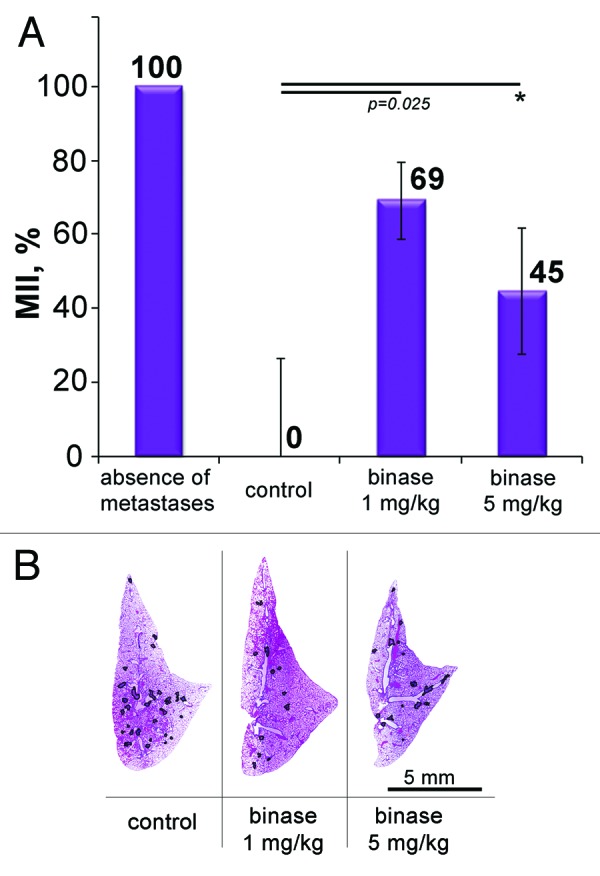
Figure 4. The effect of binase on the metastasis development in mice with metastatic model of melanoma B-16. (A) Metastasis inhibition index (MII) in the control and experimental groups. MII = [mean metastasisareacontrol − mean metastasis areaexperiment]/mean metastasisareacontrol × 100%. (B) Representative histotopograms of the lung lobes of mice that received intraperitoneal injections of binase thrice a week at the doses of 1 and 5 mg/kg. Haematoxylin and eosin staining. Borders of metastases are outlined by black line. p value indicates a statistically reliable difference relative to the control group, and (*) denotes no statistically significant difference.
Treatment with binase caused 2–3-fold increase of IL-1α from 39 pg/ml in the control group to 97 and 125 pg/ml in the groups treated with binase at the doses of 1 and 5 mg/kg, respectively (Fig. 3C). It should be noted that a similar increase of IL-1α level was also observed on LLC model after intraperitoneal injection of binase at the dose of 1 mg/kg (data not shown). Since both tumors LLC and RLS40 are models with the primary tumor site, the increase of IL-1α level after the binase treatment is a good prognostic sign, because this cytokine regulates T-cellular component of the antitumor response. Both CHOP monotherapy and the combined therapy with binase caused 8-fold increase of IL-1α level.
The levels of pro-inflammatory cytokines IL-6 and TNF-α, which indicates the necrotic processes in the body (particularly, in the primary tumor site) increased only after CHOP monotherapy and did not change after treatment with binase (Fig. 3D and E).
Effect of binase on metastasis development in melanoma B-16-bearing mice
Antimetastatic activity of binase was tested in a model of melanoma B-16, which, when injected into the tail vein, metastasizes into the lungs. Morphometric analysis showed that the treatment with binase at a dose of 1 mg/kg resulted in the inhibition of metastases development by 69% (Fig. 4A and B). The increase in the dose of the enzyme to 5 mg/kg did not result in a higher antimetastatic activity; moreover, even an apparent decrease of MII up to 45% was observed. Thus, similar to the case of lymphosarcoma RLS40, increase in the dose of binase up to 5 mg/kg did not intensify the observed antimetastatic effect.
In the absence of a primary tumor site in melanoma metastatic model, no statistically significant alteration of interferon response as well as no changes in IL-1α level after the treatment with binase were observed (Fig. 5A). It is worth mentioning that IL-1α level was very high in animals without binase treatment (341 pg/ml) (data not shown). However, treatment with binase caused significant decrease in pro-inflammatory IL-6 level (Fig. 5B), which may have a negative influence on the T-cellular antitumor component in the absence of the primary tumor site. Among the other tumors under study melanoma metastatic model was characterized by the increased level of TNF-α, which did not change after the treatment with binase (Fig. 5C).
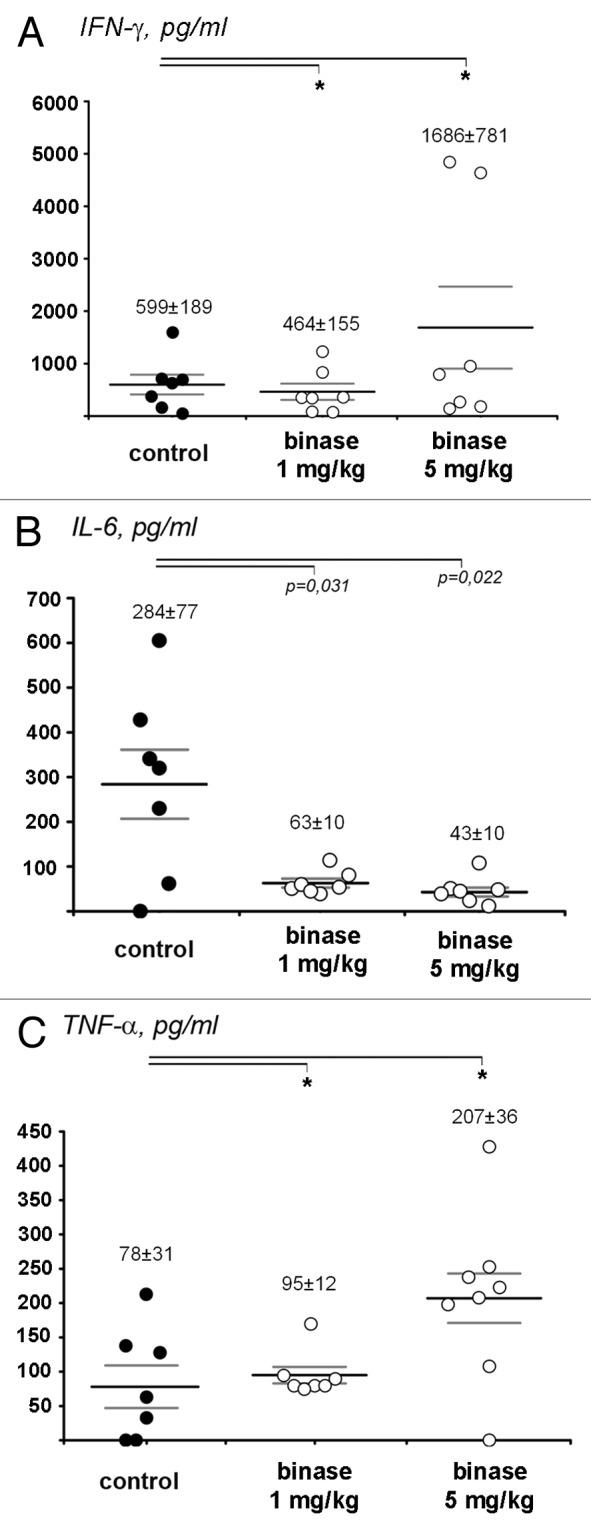
Figure 5. The change in cytokine profiles in the blood serum of mice with metastatic model of melanoma B-16 after the treatment with binase at the doses of 1 and 5 mg/kg. (A) IFN-γ, (B) IL-6, (C) TNF-α. The levels of cytokines in the blood serum were measured by ELISA. For each group of animals the value of MEAN ± SEM is shown. p value indicates a statistically reliable difference relative to the control group, and (*) denotes no statistically significant difference.
Morphological alterations in the liver of tumor bearing mice after the treatment with binase
Since most medications undergo biotransformation in the liver with the formation of toxic metabolites, morphological changes and regeneration capacity of the liver of mice treated with binase were evaluated. Upon the development of tumor or/and metastasis severe destructive changes in liver tissue were revealed. These changes amounted to 71.5 ± 3.4%, 77.9 ± 0.9% and 70.7 ± 1.6% of the entire liver parenchyma for the LLC, RLS40 and B-16 bearing mice, respectively, and were predominantly presented by necrosis. Administration of binase led to the 1.2–2.2-fold decline of destructive changes in liver parenchyma of tumor bearing mice. The numerical density of binuclear hepatocytes (reflecting regeneration capacity of the liver) of mice with LLC treated with binase intraperitoneally increased 1.2–3.0-fold in comparison with the control group, and in some cases reached the levels normal for healthy animals. In the case of the RLS40-bearing mice, the numerical density of binuclear hepatocyte decreased 1.6-fold in the groups treated with binase or with CHOP, and 4-fold in the group received combine therapy. Such severe destructive changes in the liver can be explained by the combined impact of several factors, including endogenous intoxication caused by primary tumor site, metastatic infiltration of the liver, polychemotherapy and induced cytokine production. In the case of the melanoma B-16-bearing mice the regenerative potential of liver decreases 1.7- and 1.3-fold after treatment with binase at doses of 1 and 5 mg/kg, respectively.
Effects of binase on B-16 and RLS40 cells in vitro
The antitumor and antimetastatic activity of binase observed in vivo raised questions on the mechanisms involved in binase-induced tumor cell death. To answer these questions, we performed a comprehensive analysis of the cytotoxic activity of binase toward tumor cells in vitro.
For these purposes B-16 and RLS40 cells were incubated for 48 h in the presence of 0.5 mg/ml of binase. The LLC cells were not used in these experiments, as they are maintained only by re-passaging in vivo. Binase dramatically reduces the survival of the B-16 as well as RLS40 cells through inhibition of cell respiration and reduction of their number (Fig. 6A–C). The largest decrease in the number of cells was observed in the population of B-16 cells. These cells also exhibit high sensitivity to the apoptogenic effect of binase compared with the RLS40 cells (Fig. 6D). Since the cytotoxic effect of binase is more pronounced in the B-16 cells, we used them for detailed investigation.
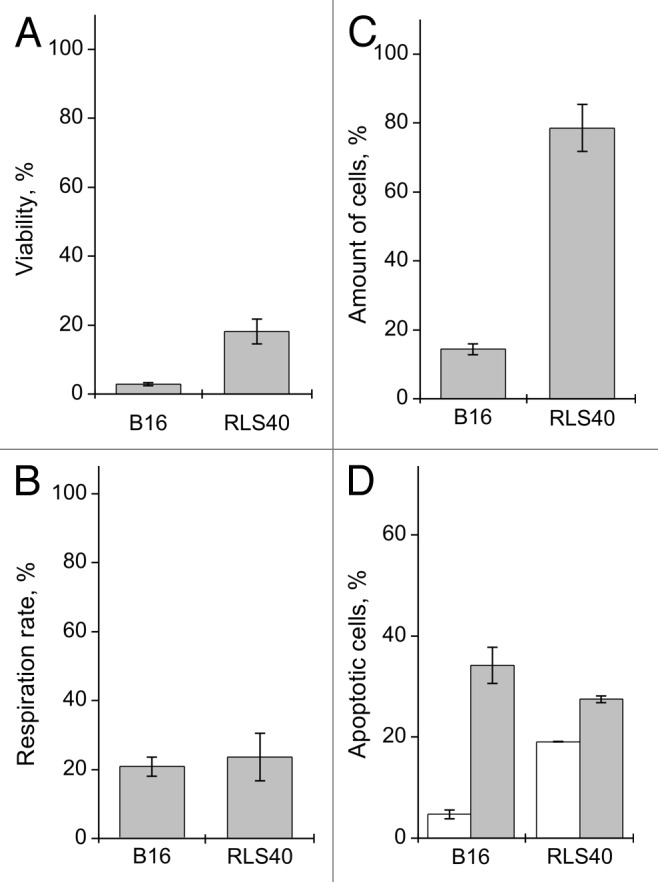
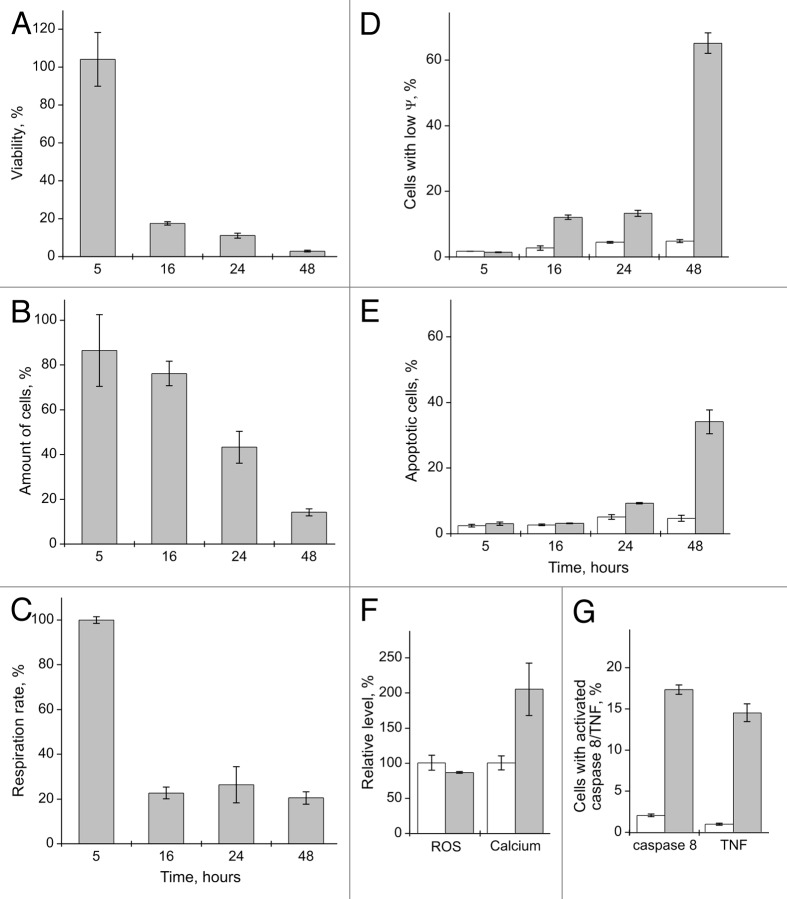
Figure 6. The effect of binase treatment (0.5 mg/ml, 48 h) on RLS40 and B-16 cells. (A) Viability, (B) proliferation rate and (C) amount of cells. Values are expressed as a percentage of the control cells without binase treatment for each cell culture. (D) Amount of apoptotic cells in the population of cells untreated and treated with binase, expressed as a percentage of the total number of cells for each variant. Each value is the mean of at least three independent experiments with triplicate samples ± SD.
After only 16 h, a significant decrease in the viability and respiration rate of the B-16 cells in comparison with control cells without binase treatment was observed, with only marginal decrease in the number of cells (Fig. 7A–C). Extending the binase treatment period to 48 h led to almost complete loss of viability of cell population (Fig. 7A).
Figure 7. The effect of binase treatment (0.5 mg/ml) on B-16 cells. (A) Viability, (B) amount of cells and (C) proliferation rate after 5, 16, 24 and 48 h of binase treatment.Values are expressed as a percentage of the control cells without binase treatment for each variant. (D) Amount of cells with low mitochondrial membrane potential (ψ) and (E) amount of apoptotic cells in untreated B-16 cells and cells treated with binase after 5, 16, 24 and 48 h, expressed as a percentage of the total number of cells for each variant. (F) Intracellular levels of Ca2+ and ROS in the untreated cells and cells treated with binase for 48 h. (G) Amount of cells with TNF on the cell surface and activated caspase 8 in untreated cells and cells treated with binase for 48 h expressed as a percentage of cells with intact membrane for each variant. Untreated cells are represented by blank columns and cells treated with binase, by filled columns. Each value is the mean of at least three independent experiments with triplicate samples ± SD.
Using a test for cell proliferation, we found that in contrast to the Kasumi-1 cells, where binase does not have cytostatic effect,26 the rate of cell proliferation of the B-16 cells drops in the presence of binase. Forty-eight h after adding binase, the cells divide on average once, whereas the control cells, three times.
The decrease in the cell respiration rate can point to the activation of the mitochondrial apoptotic pathway. To determine the mitochondrial status and apoptosis development after exposure to binase, we have estimated changes in the mitochondrial potential (ψ) and concurrently measured variation of the number of apoptotic cells in the population. Five hours after exposure to binase, no decrease was registered in the cell respiration rate, and no changes in the mitochondrial potential and the percentage of the apoptotic cells were observed. (Fig. 7C–E). Sixteen h after exposure to binase when the cell respiration rate falls, a cell population with the decreased mitochondrial potential and intact membrane emerges (12%); these cells are further characterized by the absence of a marker of apoptosis, the phosphatidylserine, on the outer membrane surface. The percentage of cells with reduced mitochondrial potential and suppression of the respiratory activity remain practically the same after 16 and 24 h of incubation with binase, but the percentage of apoptotic cells in the population begins to increase (Fig. 7E). Forty-eight h after binase exposure the percentage of cells with the reduced mitochondrial potential rises to 65%, whereas the proportion of apoptotic cells in the population is 34%. These results suggest that the effect of binase leads to the activation of the mitochondrial apoptotic pathway in the B-16 cells. The 2-fold increase of the level of intracellular Ca2+ after 48 h of the binase exposure also testifies in favor of activation of this pathway (Fig. 7F). Increase in the intracellular calcium level and decline of mitochondrial potential as a result of binase exposure was observed also in the Kasumi-1 cells.23 Similar to the Kasumi-1 cells, the level of ROS under binase exposure has a tendency to reduction (Fig. 7F), and thus ROS are not involved in the induction of apoptosis.
Earlier it has been shown that in the Kasumi-1 cells binase induces both the mitochondrial and the ligand-dependent apoptotic pathways.23 To assess the induction of the TNF-receptor ligand in the B-16 cells, the tumor necrosis factor (TNF) on the cell membrane surface was determined. After 48 h exposure to binase, the proportion of TNF-positive cells was about 15%, while there were practically no such cells in the control group. Moreover, under the effect of binase, a 15% increase in the number of intact B-16 cells with the activated caspase 8 was observed (Fig. 7G), pointing to the activation of the ligand-dependent apoptotic pathway.
Discussion
In recent years, an intensive search is being conducted for the novel therapeutic agents to treat neoplasia that on the one hand would have low toxicity for the patient and, on the other, would efficiently cause tumor cell death, preferably by the non-inflammatory way of induction of apoptosis. Among the new generation therapeutics, the enzymes of nucleic acid metabolism such as the ribonucleases attract attention; some of them are under clinical trials [ClinicalTrials.gov Identifiers: NCT01184287 (onconase); NCT01201018 and NCT01627795 (O’Shadi R, preparation containing RNase A)]. The prospects of the studies of antitumor properties of microbial RNases are explained by their abilities to provide selective apoptogenic effect on tumor cells and to evade the inhibitor of mammalian RNases. Binase is a purine-specific RNase of T1 family and a typical representative of the microbial RNases.5,15,17 Earlier the cytotoxic activity of a number of microbial RNases, including binase, was shown in vitro on different types of tumor cells,1,5,9-11,28-30 but no studies have been performed in vivo.
Here the ability of binase to retard primary tumor and metastasis growth was demonstrated for the first time using the three tumor models of different histological type having strong relevance to human tumors. The Lewis lung carcinoma LLC has epithelial origin and is related to human non-small cell lung cancer.31 Lymphosarcoma RLS40 displays the multidrug resistance phenotype, is derived from hematopoietic tissue and can be related to the human diffuse large B-cell lymphoma. Melanoma B-16 arises from the melanin-derived tissue and is related to the human metastatic melanoma.32 In our study, models were generated of the LLC and RLS40, forming primary solid tumor node and metastasizing into lungs and liver, respectively, and the metastatic model of melanoma B-16 with pulmonary metastases.
The dosage and route of administration (up to 5 mg/kg, i.p., thrice a week, during 2 wk) were selected experimentally, providing for the maximal antitumor response and no side effects. Binase administered at the dose of 1 mg/kg caused retardation of primary tumor growth up to 45% on LLC and RLS40 models, and inhibition of metastases development up to 50% on LLC and RLS40 and up to 70% on B-16 melanoma models (Figs. 1, 2 and 4). Similar efficiency of antitumor response observed upon treatment by binase of the tumors LLC and RLS40 with the multidrug resistance phenotype points to the high therapeutic potential of binase.
The effect of binase at the dose of 5 mg/kg was less pronounced (Figs. 2 and 4), probably due to the appearance of neutralizing antibodies in the blood of experimental animals, which is common and has been described for a number of protein-based drugs.
It is known that the therapeutic systemic effects in vivo depend on the histological type of tumor and the extent of its malignancy, which is extremely high in melanoma. The level of tumoricidal activity of binase in the melanoma model is comparable with the effects of some experimental chemotherapeutics, for example, inhibitor of the MAPK-interacting kinases,33 pro-apoptotic inductor diindolylmethanechimeric,34 antibodies against PRL-335 and the resorcinylicisoxazole/pyrazole HSP90 inhibitors,36 which demonstrate the ability to suppress tumor and metastasis growth in the nude mice with xerograph melanoma.
A comparison of the binase efficacy in vivo with the effect of other cytotoxic RNases shows the following. In rats with the Lewis lung carcinoma, BS-RNase (injection at a dose of 10 or 20 mg/kg every 3 d for 20 d) caused inhibition of tumor growth with efficiency 30 and 66% under the doses of BS-RNase 10 and 20 mg/kg, respectively and reduced the number of metastasis by 52%.37 The onconase at the dose of 10 mg/kg produced a 50% retardation of tumor growth in the model of human prostatic tumor xenografts in nude mice.38 Binase causes a similar antitumor effect (30–45% inhibition of the tumor growth) at a dose smaller by an order of magnitude (0.5–1 mg/kg).
No enhancement of the therapeutic effect of the combined therapy binase + CHOP on the progression of RLS40 tumor in comparison with CHOP alone was observed (Fig. 2). However, in these experiments, a positive outcome of binase treatment was found, consisting in the increase of the level of IFN-γ in blood serum and decrease of the destructive and necrotic changes in the liver. It should be noted that polychemotherapy has a toxic effect and induces the synthesis of pro-inflammatory cytokines, which is not observed in the case of treatment by binase. Binase, when administered alone, does not increase the levels of pro-inflammatory cytokines IL-6 and TNF-α and only slightly affects the level of IL-1-α (Fig. 3). The positive immunomodulatory effect of binase was also observed in the melanoma model and was manifested in the increase of IFN-γ level and sharp decrease of the level of IL-6 (Fig. 5B).
The development of all studied tumors leads to severe destructive changes in the liver and reduces its regeneration capacity. Notably, binase itself at the studied dose range did not exhibit general toxicity and hepatotoxicity in healthy mice. Treatment with binase not only exacerbates the toxic burden of tumor on liver, but reduces it and leads to the normalization of hepatic regeneration potential.
The search for molecular mechanism of cytotoxic effect of binase on tumor cells was performed using cell lines related to tumor models studied in vivo. Despite the fact that binase similarly suppressed respiration of the B-16 and RLS40 cells, the proportion of apoptotic cells in the population of B-16 was significantly higher (Fig. 6). Due to the greater sensitivity of B-16 cells to binase, this cell line was used for elucidation of binase-activated apoptotic pathways. One of the first responses of a tumor cell to binase exposure is the drop in the mitochondrial potential and increase of the intracellular Ca2+ level, leading to the development of apoptosis via the intrinsic apoptotic pathway. Subsequently, the TNF-α is synthesized determining the startup of extrinsic apoptotic pathway through the binding of TNF with the cell-death receptors and activation of caspase 8 (Fig. 7).
Thus, the antitumor activity of binase is realized via induction of the intrinsic and extrinsic apoptotic pathways. Similar induction of apoptotic pathways by binase treatment was demonstrated recently for mielogenic leukemia Kasumi-1 cells.26 Cytotoxic effect of binase on the RLS40 cells is manifested in a similar way, and a lower percentage of apoptotic cells in a population of the RLS40 cells may be associated with increased expression of the genes bcl-2 and mdr-1b.27 Obtained data shows that there is a direct correlation between the sensitivity of tumor cell line in vitro and the response of the relevant tumor to treatment in vivo.
It is clear that the nature of impact of binase on tumor cells in vivo is more complex and incorporates, in addition to the apoptotic effect on tumor cells, systemic response of the organism, including activation of antitumor immune response (for example, increase of the level of INF-γ) and “hepatoprotective” effects. Due to the fact that there is evidence of the presence of binase in the bloodstream 15 min after injection (Fig. S1), the potential ability of binase to change the pattern of small regulatory RNAs cannot be excluded. The latter assumption was put forward for some other antitumor ribonucleases.13,21,35,36,39,40
Our results unambiguously show that binase therapy on animals bearing tumors of different histological types leads to effective retardation of primary tumor and metastasis growth and along with it has general systemic, immunomodulatory and “hepatoprotective” effects. One of the important results is that binase causes cytotoxic effect on tumor cells through the induction of apoptosis but does not induce the development of necrotic events and inflammatory response in the organism. Tumors that responded well to binase treatment in vivo have strong relevance to the human analogs, suggesting the therapy with binase has prospects as adjuvant treatment for high-risk metastasizing human neoplasia.
Materials and Methods
Binase preparation and purification
Binase (12.3 kDa) was isolated as a homogenous protein from the cultural fluid of Escherichia coli BL21 cells carrying plasmid pGEMGX1/ent/Bi. The enzyme was purified as described earlier.41 Endotoxins content in binase preparations, determined by the Limulus amoebocyte lysate test (LAL) (Charles River Endosafe), was less than 5 EU/mg. Binase was assayed for catalytic activity using poly(I) as substrate.17
Cell cultures
B-16, the C57Bl/6J-derived melanoma cells, were obtained from the Institute of Cytology (RAS). The modified RLS40 cells were from cell collection of the Institute of Chemical Biology and Fundamental Medicine (SB RAS). LLC cells were generously provided by Dr N.A. Popova (Institute of Cytology and Genetics, SB RAS). B-16 and RLS40 cells were grown on DMEM and IMDM media, respectively, containing 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mМ glutamine at 37°C in a humid atmosphere with 5% CO2.
Determination of proliferation rate, apoptosis, mitochondrial membrane potential, intracellular levels of ROS and Ca2+ and levels of activated caspase 8 by flow cytometry
CellTrace Violet Cell Proliferation Kit (Invitrogen) was used for analysis of cell proliferation according to Mitkevich et al.26 Cells with damaged membranes were detected by propidium iodide (PI) (Sigma).23 Apoptosis was analyzed by double staining with Annexin V-FITC (Invitrogen)42 and PI.43 Mitochondrial membrane potential (Ψ) was detected by MitoProbeDilC1(5) (Ex/Em 638/658 nm) (Invitrogen). Cells (1 × 106) were incubated with 0.5 μM DilC1(5) for 30 min at 37°C in darkness. Cells were then washed with PBS at 4°C and resuspended with PBS. ROS and Ca2+ levels were estimated by staining with H2DCF-DA (Ex/Em 485/525 nm) and fluo-4 (Ex/Em 494/516 nm) (Invitrogen), correspondingly, according to Mitkevich et al.26 Cells with active caspase 8 were detected using Vybrant FAM™ caspase-8 assay kit (Ex/Em 495/529 nm) (Invitrogen) according to the manufacturer’s protocol.
Viability and respiration rate of cells
Cell viability and respiration rate were assessed with a WST-1-based test (Roche Diagnostics) as described earlier.23
Tumor transplantation and design of animal experiments
All animal procedures were performed in compliance with the approved protocols and recommendations for proper use and care of laboratory animals [ECC Directive 86/609/EEC]. Ten- to 12-wk-old female C57Bl/6 and CBA/LacSto mice were used in the experiments. Solid tumors LLC or RLS40were induced by intramuscular injection of LLC or RLS40 cells (106) suspended in 0.1 ml of saline buffer into the right thighs of C57Bl/6 and CBA/LacSto mice, respectively. To generate a metastatic model of melanoma B-16 tumor cells (105) suspended in 0.2 ml of saline buffer were inoculated into the lateral tail vein of C57Bl/6 mice.
LLC-bearing mice were treated by intramuscular or intraperitoneal administration of binase at doses of 0.1, 0.5 and 1 mg/kg, starting on day 4 after tumor transplantation. A total number of eight injections within 2 wk was administered. RLS40-bearing mice and mice with metastatic model of B-16 were treated by intraperitoneal administration of binase at doses of 1 and 5 mg/kg thrice a week within 2 wk, starting on day 4 after tumor transplantation.
The effect of combined treatment with binase and CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) was studied in the experiment with RLS40-bearing mice. CHOP treatment was administered once a week on day 4 and 11 after tumor transplantation. Standard combination of cytostatics (according to CHOP protocol), including cyclophosphamide, doxorubicin, vincristine and prednisolone, was used. The cytostatics were dissolved in the saline buffer directly before use and injected in doses corresponding to 1/5 of LD50: cyclophosphamide (50 mg/kg), doxorubicin (4 mg/kg), vincristine (0.1 mg/kg) (Lens-Farm) and prednisolone (5 mg/kg) (Nycomed). Doxorubicin was injected into the caudal vein; other cytotoxic agents were injected intraperitoneally.
Tumor size was determined every 2 d by caliper measurements in three perpendicular dimensions. Tumor volumes were calculated as V = (π/6 × length × width × height). At the end of the experiments, in 20 min after the last injection of binase, blood samples from mice were collected to perform ELISA assays and measurements of binase activity, and lungs and livers were collected for histology and morphometry.
Binase toxicity on healthy mice
Binase toxicity was studied on healthy animals in C57Bl/6 and CBA/LacSto mice. C57Bl/6 and CBA mice were treated by intraperitoneal administration of binase at doses of 1 and 5 mg/kg thrice a week during 2 wk. Control groups included intact animals that received no injections and animals with intraperitoneal injections of saline buffer thrice a week during 2 wk. In the experiment, the body weight of mice, condition of the hair and skin, the consumption of food and water were evaluated. Material for histological study (livers) was collected on day 14 after initiation of treatment.
Measurement of cytokine levels in the blood serum
Blood serum was prepared by clot formation at 37°C for 30 min and at 4°C overnight followed by clot discard and serum centrifugation (4,000 rpm, 4°C, 20 min). Serum samples were stored at −70°C until analysis. The levels of TNF-α, IL-6, IL-1α and IFN-γ in blood serum of mice were measured using Mouse IFN gamma, IL-6, IL-1-α and TNF α Colorimetric ELISA Kits (Thermo Scientific) according to the manufacturer’s protocols. The level of IFN-α was measured using Mouse Interferon Alpha (Mu-IFN-) ELISA Kit (Thermo Scientific).
Histology and morphometry
For histological and morphometric analysis, the lungs and livers were fixed in 10% neutral-buffered formalin, routinely processed and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin, microscopically examined and scanned. The percentage of the metastases area in relation to the area of the measured lobe of the examined organ was calculated. The metastasis inhibition index was calculated as MII = [mean metastasis areacontrol – mean metastasis areaexperiment]/mean metastasis areacontrol × 100%. The MII of the control group reflects the absence of metastasis inhibition and is equal to 0%.
To assess the toxic effects of polychemotherapy and binase, stereological quantification of the liver samples was performed by point counting, using closed test-system at a magnification 40×. Used test-system has 81 straight-line segments and 100 testing points in a testing area equal to 3.2 × 106 μm2.Ten or 15 random fields were studied in each specimen, in total forming 100–150 fields for each group of mice. The volume densities (Vv) of normal liver parenchyma, hepatocytes with degenerate and necrotic changes and numerical density (Nv) of binuclear hepatocytes reflecting the regeneration capacity of the liver were evaluated as described elsewhere.44
Statistics
The data were statistically processed using the Student’s t-test (two-tailed, unpaired); a p value of ≤ 0.05 was considered to indicate a significant difference.
Supplementary Material
Acknowledgments
We thank Ksenia Burnysheva for assistance in the measurements of the enzyme activity, Albina Vladimirova for cell maintenance and Alexandra Moznay for help in experiments with animals. This work was supported by the Molecular and Cellular Biology Program and by the Program “Fundamental sciences to medicine 2012-24” of the Russian Academy of Sciences, by Scientific Schools (#2972.2012.4), by grant from the Russian Foundation for Basic Research (#12-04-33248), and by ICGEB (grant CRP/RUS11-02). The part of the work was performed on the equipment of center “Genome,” which supported by State Contract #16.552.11.7069 with the Russian Ministry of Education and Science.
Glossary
Abbreviations:
- LLC
Lewis lung carcinoma
- RNase
ribonuclease
- binase
Bacillus intermedius RNase
- MII
metastasis inhibition index
- CHOP
conventional polychemotherapy
- Ψ
mitochondrial membrane potential
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- PI
propidium iodide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/25164
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25164
References
- 1.Leland PA, Raines RT. Cancer chemotherapy--ribonucleases to the rescue. Chem Biol. 2001;8:405–13. doi: 10.1016/S1074-5521(01)00030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardelt W, Ardelt B, Darzynkiewicz Z. Ribonucleases as potential modalities in anticancer therapy. Eur J Pharmacol. 2009;625:181–9. doi: 10.1016/j.ejphar.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang EF, Ng TB. Ribonucleases of different origins with a wide spectrum of medicinal applications. Biochim Biophys Acta. 2011;1815:65–74. doi: 10.1016/j.bbcan.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Edelweiss E, Balandin TG, Ivanova JL, Lutsenko GV, Leonova OG, Popenko VI, et al. Barnase as a new therapeutic agent triggering apoptosis in human cancer cells. PLoS One. 2008;3:e2434. doi: 10.1371/journal.pone.0002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarov AA, Kolchinsky A, Ilinskaya ON. Binase and other microbial RNases as potential anticancer agents. Bioessays. 2008;30:781–90. doi: 10.1002/bies.20789. [DOI] [PubMed] [Google Scholar]
- 6.Kotchetkov R, Cinatl J, Krivtchik AA, Vogel JU, Matousek J, Pouckova P, et al. Selective activity of BS-RNase against anaplastic thyroid cancer. Anticancer Res. 2001;21(2A):1035–42. [PubMed] [Google Scholar]
- 7.Cinatl J, Jr., Cinatl J, Kotchetkov R, Vogel JU, Woodcock BG, Matousek J, et al. Bovine seminal ribonuclease selectively kills human multidrug-resistant neuroblastoma cells via induction of apoptosis. Int J Oncol. 1999;15:1001–9. doi: 10.3892/ijo.15.5.1001. [DOI] [PubMed] [Google Scholar]
- 8.Poucková P, Zadinová M, Hlousková D, Strohalm J, Plocová D, Spunda M, et al. Polymer-conjugated bovine pancreatic and seminal ribonucleases inhibit growth of human tumors in nude mice. J Control Release. 2004;95:83–92. doi: 10.1016/j.jconrel.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Lee JE, Raines RT. Cytotoxicity of bovine seminal ribonuclease: monomer versus dimer. Biochemistry. 2005;44:15760–7. doi: 10.1021/bi051668z. [DOI] [PubMed] [Google Scholar]
- 10.Lee JE, Raines RT. Ribonucleases as novel chemotherapeutics : the ranpirnase example. BioDrugs. 2008;22:53–8. doi: 10.2165/00063030-200822010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee I, Lee YH, Mikulski SM, Lee J, Covone K, Shogen K. Tumoricidal effects of onconase on various tumors. J Surg Oncol. 2000;73:164–71. doi: 10.1002/(SICI)1096-9098(200003)73:3<164::AID-JSO10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Costanzi J, Sidransky D, Navon A, Goldsweig H. Ribonucleases as a novel pro-apoptotic anticancer strategy: review of the preclinical and clinical data for ranpirnase. Cancer Invest. 2005;23:643–50. doi: 10.1080/07357900500283143. [DOI] [PubMed] [Google Scholar]
- 13.Patutina O, Mironova N, Ryabchikova E, Popova N, Nikolin V, Kaledin V, et al. Inhibition of metastasis development by daily administration of ultralow doses of RNase A and DNase I. Biochimie. 2011;93:689–96. doi: 10.1016/j.biochi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Nitta K, Ozaki K, Ishikawa M, Furusawa S, Hosono M, Kawauchi H, et al. Inhibition of cell proliferation by Rana catesbeiana and Rana japonica lectins belonging to the ribonuclease superfamily. Cancer Res. 1994;54:920–7. [PubMed] [Google Scholar]
- 15.Ilinskaya O, Decker K, Koschinski A, Dreyer F, Repp H. Bacillus intermedius ribonuclease as inhibitor of cell proliferation and membrane current. Toxicology. 2001;156:101–7. doi: 10.1016/S0300-483X(00)00335-8. [DOI] [PubMed] [Google Scholar]
- 16.Sevcik J, Urbanikova L, Leland PA, Raines RT. X-ray structure of two crystalline forms of a streptomycete ribonuclease with cytotoxic activity. J Biol Chem. 2002;277:47325–30. doi: 10.1074/jbc.M208425200. [DOI] [PubMed] [Google Scholar]
- 17.Mitkevich VA, Tchurikov NA, Zelenikhin PV, Petrushanko IY, Makarov AA, Ilinskaya ON. Binase cleaves cellular noncoding RNAs and affects coding mRNAs. FEBS J. 2010;277:186–96. doi: 10.1111/j.1742-4658.2009.07471.x. [DOI] [PubMed] [Google Scholar]
- 18.Arnold U, Ulbrich-Hofmann R. Natural and engineered ribonucleases as potential cancer therapeutics. Biotechnol Lett. 2006;28:1615–22. doi: 10.1007/s10529-006-9145-0. [DOI] [PubMed] [Google Scholar]
- 19.Rutkoski TJ, Kink JA, Strong LE, Raines RT. Site-specific PEGylation endows a mammalian ribonuclease with antitumor activity. Cancer Biol Ther. 2011;12:208–14. doi: 10.4161/cbt.12.3.15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutkoski TJ, Kink JA, Strong LE, Schilling CI, Raines RT. Antitumor activity of ribonuclease multimers created by site-specific covalent tethering. Bioconjug Chem. 2010;21:1691–702. doi: 10.1021/bc100292x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao M, Zu LD, He XH, Shen RL, Wang QC, Liu MF. Onconase downregulates microRNA expression through targeting microRNA precursors. Cell Res. 2012;22:1199–202. doi: 10.1038/cr.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilinskaya ON, Zelenikhin PV, Petrushanko IY, Mitkevich VA, Prassolov VS, Makarov AA. Binase induces apoptosis of transformed myeloid cells and does not induce T-cell immune response. Biochem Biophys Res Commun. 2007;361:1000–5. doi: 10.1016/j.bbrc.2007.07.143. [DOI] [PubMed] [Google Scholar]
- 23.Mitkevich VA, Petrushanko IY, Spirin PV, Fedorova TV, Kretova OV, Tchurikov NA, et al. Sensitivity of acute myeloid leukemia Kasumi-1 cells to binase toxic action depends on the expression of KIT and АML1-ETO oncogenes. Cell Cycle. 2011;10:4090–7. doi: 10.4161/cc.10.23.18210. [DOI] [PubMed] [Google Scholar]
- 24.Mitkevich VA, Orlova NN, Petrushanko IYu, Simonenko OV, Spirin PV, Prokofieva MM, et al. Expression of FLT3-ITD oncogene confers mice progenitor B-cells BAF3 sensitivity to the ribonuclease binase cytotoxic action. Mol Biol (Mosk) 2013;47:249–52. doi: 10.1134/S002689331302009X. [DOI] [PubMed] [Google Scholar]
- 25.Mitkevich VA, Petrushanko IY, Kretova OV, Zelenikhin PV, Prassolov VS, Tchurikov NA, et al. Oncogenic c-kit transcript is a target for binase. Cell Cycle. 2010;9:2674–8. doi: 10.4161/cc.9.13.12150. [DOI] [PubMed] [Google Scholar]
- 26.Mitkevich VA, Kretova OV, Petrushanko IY, Burnysheva KM, Sosin DV, Simonenko OV, et al. Ribonuclease binase apoptotic signature in leukemic Kasumi-1 cells. Biochimie. 2013;95:1344–9. doi: 10.1016/j.biochi.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Mironova N, Shklyaeva O, Andreeva E, Popova N, Kaledin V, Nikolin V, et al. Animal model of drug-resistant tumor progression. Ann N Y Acad Sci. 2006;1091:490–500. doi: 10.1196/annals.1378.090. [DOI] [PubMed] [Google Scholar]
- 28.Matousek J. Ribonucleases and their antitumor activity. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:175–91. doi: 10.1016/S1532-0456(01)90202-9. [DOI] [PubMed] [Google Scholar]
- 29.Makarov AA, Ilinskaya ON. Cytotoxic ribonucleases: molecular weapons and their targets. FEBS Lett. 2003;540:15–20. doi: 10.1016/S0014-5793(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 30.Ardelt W, Shogen K, Darzynkiewicz Z. Onconase and amphinase, the antitumor ribonucleases from Rana pipiens oocytes. Curr Pharm Biotechnol. 2008;9:215–25. doi: 10.2174/138920108784567245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakanishi H, Takenaga K, Oguri K, Yoshida A, Okayama M. Morphological characteristics of tumours formed by Lewis lung carcinoma-derived cloned cell lines with different metastatic potentials: structural differences in their basement membranes formed in vivo. Virchows Arch A Pathol Anat Histopathol. 1992;420:163–70. doi: 10.1007/BF02358808. [DOI] [PubMed] [Google Scholar]
- 32.Bobek V, Kolostova K, Pinterova D, Kacprzak G, Adamiak J, Kolodziej J, et al. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 2010;30:4799–803. [PubMed] [Google Scholar]
- 33.Konicek BW, Stephens JR, McNulty AM, Robichaud N, Peery RB, Dumstorf CA, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–57. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 34.Kandala PK, Srivastava SK. Regulation of macroautophagy in ovarian cancer cells in vitro and in vivo by controlling glucose regulatory protein 78 and AMPK. Oncotarget. 2012;3:435–49. doi: 10.18632/oncotarget.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo K, Tang JP, Jie L, Al-Aidaroos AQ, Hong CW, Tan CP, et al. Engineering the first chimeric antibody in targeting intracellular PRL-3 oncoprotein for cancer therapy in mice. Oncotarget. 2012;3:158–71. doi: 10.18632/oncotarget.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–60. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 37.Laccetti P, Spalletti-Cernia D, Portella G, De Corato P, D’Alessio G, Vecchio G. Seminal ribonuclease inhibits tumor growth and reduces the metastatic potential of Lewis lung carcinoma. Cancer Res. 1994;54:4253–6. [PubMed] [Google Scholar]
- 38.Lee I, Lee YH, Mikulski SM, Lee J, Covone K, Shogen K. Tumoricidal effects of onconase on various tumors. J Surg Oncol. 2000;73:164–71. doi: 10.1002/(SICI)1096-9098(200003)73:3<164::AID-JSO10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Ardelt B, Ardelt W, Darzynkiewicz Z. Cytotoxic ribonucleases and RNA interference (RNAi) Cell Cycle. 2003;2:22–4. doi: 10.4161/cc.2.1.232. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H, Ardelt B, Ardelt W, Shogen K, Darzynkiewicz Z. The cytotoxic ribonuclease onconase targets RNAi (siRNA) Cell Cycle. 2008;20:3258–61. doi: 10.4161/cc.7.20.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulga A, Kurbanov F, Kirpichnikov M, Protasevich I, Lobachov V, Ranjbar B, et al. Comparative study of binase and barnase: experience in chimeric ribonucleases. Protein Eng. 1998;11:775–82. doi: 10.1093/protein/11.9.775. [DOI] [PubMed] [Google Scholar]
- 42.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 43.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 44.Sen'kova AV, Mironova NL, Patutina OA, Ageeva TA, Zenkova MA. The toxic effects of polychemotherapy onto the liver are accelerated by the upregulated MDR of lymphosarcoma. ISRN Oncol 2012; 721612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.