Abstract
T lymphoma invasion and metastasis protein (Tiam1) is up-regulated in variety of cancers and its expression level is related to metastatic potential of the type of cancer. Earlier, Tiam1 was shown to be overexpressed in retinoblastoma (RB) and we hypothesized that it was involved in invasiveness of RB. This was tested by silencing Tiam1 in RB cell lines (Y79 and Weri-Rb1) using siRNA pool, targeting different regions of Tiam1 mRNA. The cDNA microarray of Tiam1 silenced cells showed gene regulations altered by Tiam1 were predominantly on the actin cytoskeleton interacting proteins, apoptotic initiators and tumorogenic potential targets. The silenced phenotype resulted in decreased growth and increased apoptosis with non-invasive characteristics. Transfection of full length and N-terminal truncated construct (C1199) clearly revealed membrane localization of Tiam1 and not in the case of C580 construct. F-actin staining showed the interaction of Tiam1 with actin in the membrane edges that leads to ruffling, and also imparts varying invasive potential to the cell. The results obtained from our study show for the first time that Tiam1 modulates the cell invasion, mediated by actin cytoskeleton remodeling in RB.
Introduction
Retinoblastoma (RB) is an intraocular tumor of childhood. As per International Retinoblastoma Staging Working Group (IRSWG) most of the RB tumors are found to have massive choroidal, optic nerve, and anterior segment invasion [1]. The risk factors include choroidal invasion >3 mm (CI>3 mm), post laminar and surgical end of optic nerve invasion. Understanding the molecular regulation of tumor cell invasion and apoptosis helps in identifying new therapeutic targets.
T lymphoma invasion and metastasis protein (Tiam1) was first identified as an invasion and metastasis inducing gene using T lymphoma cells by proviral tagging and in vitro selection for invasiveness [2], [3]. Tiam1 is a guanine nucleotide exchange factor (GEF) that mediates the specific activation of Rac1 [4], [5], [6]. Small guanine triphosphate (GTP) binding proteins belonging to Ras superfamily act as molecular switches for activation of cellular activities such as signal transduction, actin cytoskeleton remodeling, microtubule stabilization, centrosome reorganization and intracellular trafficking [7], [8], [9], [10]. Aberrations or mutations of these proteins lead to malignancy of the cell. In response to extracellular signals, GEFs play a major role by catalyzing the activation of GTP-binding proteins by dissociation of guanosine diphosphate bound to it. RhoA, Rac1 and Cdc42 are key proteins of Rho family that depends on GEFs for their acitivation [11], [12].
Tiam1 has been linked with cancer progression and having growth promoting functions based on the tumor type. Overexpression of N-terminus truncated Tiam1 is found to impart oncogenic activity in NIH 3T3 cells [13], [14]. Similarly, mutations in Tiam1 gene are able to transform NIH 3T3 cells [15]. Oncogenic potential of Tiam1 was found to be present in various tumors with respect to the tumor grade and stage. The over expression of Tiam1 in breast carcinoma, nasopharyngeal carcinoma, hepatocellular carcinoma, renal cell carcinoma, retinoblastoma, colorectal carcinoma, lung and prostate cancer has been previously reported [16], [17], [18], [19], [20], [21], [22]. Tiam1 is negatively correlated in case of renal carcinoma, where it inhibits invasion by promoting E-cadherin mediated adhesion [15].
Tiam1 contains consensus myristoylation sequence at the amino terminus, two PEST sequences, a Ras binding domain (RBD), PSD-95/DlgA/ZO-1 domain (PDZ), two pleckstrin homology (PH) domains and DH domain [23], [24], [25] (Figure 1A). The PH domain present in carboxy terminal next to DH domain is similar in all other GEFs. The DHR domain is important for the protein-protein interaction [26]. Presence of Tiam1 on the membrane surface is accomplished through the N-terminal PH domain, and not by the c-terminal PH domain or DHR domain. Using truncated constructs of Tiam1, localization of Tiam1 in the membrane is shown to be necessary for the membrane ruffling [24], [27], [28].
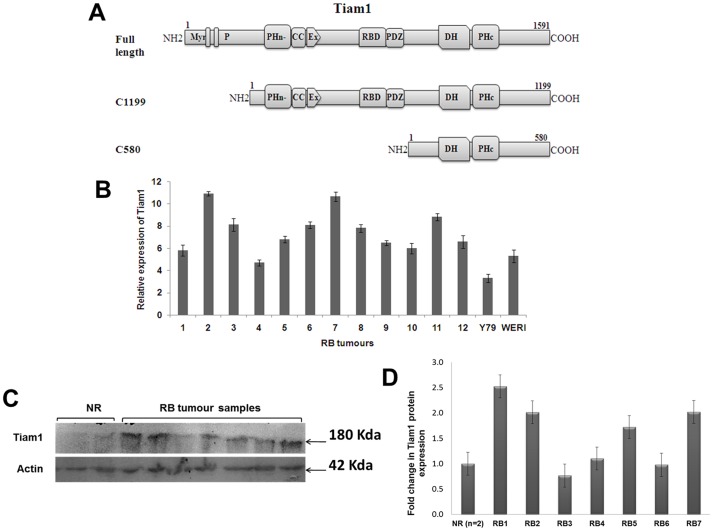
Figure 1. Schematic representation of Tiam1 constructs and expression level of Tiam1 in RB tumors compared to normal retina.
A. Full length Tiam1 consists of 1591 aminoacids. Tiam1 has several specific domains such as, Myr: Myristoylation site; P: PEST sequences; PHn: N-terminal PH domain; CC: Coiled-coil region; Ex: Extended structure; RBD: Ras-binding domain; PDZ: PSD-95/DlgA/ZO-1 domain; DH: Dbl homology domain and PHc: C-terminal PH domain. Deletion of N-terminal myr and P sites leaves the active C1199 form of Tiam1 in truncated constructs. C580 contains only DH and C-terminal PH domain. B. Fold difference in the expression of Tiam1 mRNA in RB tumors versus normal retina by qPCR analysis. The relative fold change (log2 ratio) in Tiam1 mRNA expression is calculated by normalizing with β2-microglobulin using normal retina as control sample. C. Western blot analysis of Tiam1 protein expression in RB tumors and normal retina shows Tiam1 at 180 kda and β-actin at 42 Kda. D. Densitometry analysis of the Tiam1 protein expression in RB tumors vs normal retina (n = 2). RB1 to RB7 invidividual expression normalized with averaged NR is represented.
Earlier, we showed the expression of Rho, Rac, Cdc42 and Tiam1 in RB. Additional studies on E-Cadherin and N-Cadherin has helped us to correlate the expression levels of these antigens with respect to the function they mediate in the RB tumor progression. Especially the expression of Tiam1 was positively correlated with the invasive potential of the tumors [18]. The functional relevance behind this overexpression of Tiam1 in tumorogenesis and invasion of RB is not yet elucidated. In the current study, we analyzed the effect of Tiam1 on cell proliferation and invasion using RB cell lines, Y79 and Weri-Rb1. Additionally, we addressed the effects of the truncated constructs with respect to subcellular localization and F-actin based interaction and invasiveness attributed to the RB cell lines.
Materials and Methods
Cell culture
Human RB cell lines Y79 and Weri-Rb1 were obtained from the cell bank, RIKEN BioResource Center (Ibaraki, Japan). Roswell Park Memorial Institute (RPMI) 1640 media, Heat- inactivated Fetal Bovine Serum (FBS), Poly-L-Lysine (PLL) were purchased from Sigma Aldrich (St. Louis, MO). The cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, India) along with 1% Pen-strep (Hi-Media, Mumbai, India). The cells were maintained in 5% CO2 incubator at 37°C.
Sample collection and Ethics statement
Tumors samples were collected from enucleated eyeballs of RB patients as a part of treatment with written consent from patient/guardian and utilized for research purpose anonymously. Retina samples were collected from normal cadaveric donor eyeballs from C U shah eye bank, Medical Research Foundation, Sankara Nethralaya, India. This work was approved by Vision Research Foundation ethics committee and done at Vision Research Foundation, Sankara Nethralaya (Ethical clearance no: 136-2008-P).
Transfection and RNA interference of Tiam1
Full-length and N-terminal truncated C1199 and C580 Tiam1 containing a hemagglutinin tag at the 3′ end, cloned in the eukaryotic expression vector pUTSV1 (Eurogentec, Belgium) were procured from John G Collard, Netherlands Cancer Institute [13]. Tiam1 constructs were transiently transfected in Y79 and Weri-Rb1 cell lines with LipofectAMINE 2000 (Invitrogen) as prescribed in manufacture's protocol. For Tiam1 silencing, three pre-designed short interfering RNA (siRNA) sequences targeting different regions of Tiam1 mRNA were purchased from Qiagen, Valencia, USA. The siRNA sequences are as follows, GGCGAGCUUUAAGAAGAAATT (sense) and UUUCUUCUUAAAGCUCGCCGT (antisense) for Tiam1_1 (T1), CAUGUAGAGCACGAGUUUUTT (sense) and AAAACUCGUGCUCUACAUGTT (antisense) for Tiam1_5 (T5), GGUUCUGUCUGCCCAAUAATT (sense) and UUAUUGGGCAGACAGAACCAG (antisense) for Tiam1_6 (T6). Briefly, the cells were transfected with 200 nM of pooled siRNA sequences by using LipofectAMINE 2000. Scrambled siRNA was used as a negative control in all experiments. The cells were collected after 48 hrs for further experiments.
Total RNA isolation and Quantitative PCR
The total mRNA was isolated from transfected cells and fresh RB tumors by RNAeasy kit (Qiagen, Valencia, USA) and Trizol respectively. 1 µg of total RNA was transcribed into cDNA using oligo-dT and random hexamers. The quantitative PCR was performed in Applied Biosystem 7500 by using Sybr-green (Thermoscientific, Mumbai, India). The primer sequences for Tiam1, 18S and β2M were as follows, FP: 5′AAGACGTACTCAGGCCATGTCC-3′and RP: 5′-GACCCAAATGTCGCAGTCAG-3′ for Tiam1, FP: 5′- AACCCGTTGAACCCCATT-3′ and RP: 5′-CCATCCAATCGGTAGTAG-3′ for 18S, and FP: 5′-TATCCAGCGTACTCCAAAGA-3′ and RP: 5′- GACAAGTCTGAATGCTCCAC –3′ for β2M. Comparative quantification (normal retina vs primary RB tumors or untreated cells vs Tiam1 siRNA treated cells) was determined using the formula 2−ΔΔCt and relative expression values normalized to the 18S or β2M endogenous control were used for plotting. Experiments were performed in triplicate for the same samples.
Western Blotting
Transfected cells and RB tumors were collected and washed with 1X PBS followed by cell lysis in RIPA buffer (containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 1%Triton X-100, 1% Sodium deoxycholate, 0.1% SDS and Protease inhibitor cocktail, Sigma). 50–100 µg of protein lysate was resolved on 8% SDS Polyacrylamide gel and then electroblotted onto nitrocellulose membrane at 100V for 90 min. The membrane was blocked in 5% skimmed milk, further incubated with primary anti-Tiam1 polyclonal antibody (raised in rabbit, C-16, Santacruz) in 1∶100 dilutions and anti-β actin monoclonal antibody (raised in mouse, Sigma) in 1∶2000 at 4°C for overnight. The membrane was washed thrice with TBST (1 M Tris-HCl pH-7.6 containing 0.1% Tween-20 and 0.8% NaCl) and incubated with HRP-conjugated secondary antibody at 1∶2500 dilution for 2 hrs. The membrane was detected by supersignal west femto maximum sensitivity substrate (Thermoscientific, Rockford, USA). The blots are representative of three experiments.
Immunofluorescence
For immunofluorescence, cells were seeded on Poly-L-Lysine coated cover slips in 24-well plate. After 48 hrs of transfection, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and blocked with 5% BSA in PBS for 30 mins. The cells were incubated with polyclonal anti-Tiam1 antibody (1∶25) for overnight at 4°C. After PBS wash cells were incubated with FITC conjugated anti-rabbit secondary antibody (1∶500) for 2 hrs at room temperature then incubated with TRITC conjugated phalloidin at 1∶300 dilution (Sigma Aldrich, St. Louis, MO) for 30 min whereas DAPI was used as a nuclear stain. The cover slips were mounted and viewed under Axio Observer fluorescence microscope (Zeiss, Germany).
Flow cytometry analysis of apoptosis
For the apoptosis assay, Annexin V kit from BD biosciences(San Diego, CA) was used. Briefly, cells were washed with ice cold 1X PBS twice and resuspended in 1X binding buffer and incubated with annexin V-FITC and Propidium iodide for 15 mins, followed by flow cytometry. Experiments were performed thrice individually.
Cell proliferation assay
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the percentage viability of silenced cells. Transfected cells were incubated with 100 μl of media containing 10 μl of MTT (5 mg/ml) and incubated for 4 hrs at 37°C. Then media with MTT was removed and 100 μl of DMSO was added to each well the absorbance was measured at 570 nm. All experiments were performed in triplicate.
cDNA microarray
Whole genome microarray was performed in Tiam1 siRNA treated Y79 cells along with untransfected Y79 cells. The experiment was performed in triplicates. In brief, 500 ng of total RNA was used for cDNA synthesis, followed by amplification/labeling using TotalPrep RNA Amplification kit (Ambion Inc., Austin, TX) to synthesize biotin-labeled cRNA. The concentration of cRNA was measured by spectrophotometer (Nanodrop, ND-1000, Thermo scientific). Labeled, amplified cRNA (750 ng per array) was hybridized to a ver. 3 of the Illumina Human-Ht-12 BeadChip (48 K) according to the Manufacturer's instructions (Illumina, Inc., San Diego, CA). The whole 48803 probes on the Human-Ht12 beadChip ver. 3 were used and the arrays were scanned with an Illumina Bead array Reader confocal scanner (BeadStation 500GXDW; Illumina, Inc.). Sample Gene Profile option of Illumina BeadStudio software was used to export the gene expression data.
Wound healing assay
The cells were grown on PLL coated culture plate until confluent in 1% serum medium. A wound was made by scratching a straight line using a 200 µl pipette tip. The cells were then washed twice, transfected and incubated further for 48 hrs with 10% FBS containing media. Images were taken under Phase contrast microscope at 0 hr and 48 hr using AxioObserver fluorescent microscope.
Matrigel invasion assay
Y79 and Weri-Rb1 cells were counted 24 hr post transfection of the siTiam1 and scramble siRNA and 5×104 cells in serum free media were seeeded respectively in the rehydraded matrigel invasion chamber with 10% FBS containing media added as chemoattractant. Cells were allowed to incubate for 48 hr. Chambers were removed, washed twice with 1× PBS, cleaned the matrigel using cotton plug, fixed cells in methanol, stained with cystal violet. The membranes were cut, removed and mounted with DPX mountant and viewed under 20× objective of Axio-Observer microscope.
Statistical analysis
Statistical analysis was performed by unpaired students t-test using Graphpad software on the triplicate data generated from three individual experiments. The two-tailed P values less than 0.05 were considered as significant.
Results
Differential expression of Tiam1 in RB tumors
In our earlier study, we reported the expression of Tiam1 protein in RB tumors by immunohistochemistry. In this study, we analyzed both the mRNA and protein levels of Tiam1 through qPCR and western blotting. The differential expression of Tiam1 in RB tumors (tumors with choroid invasion (CI) <3 mm and CI>3 mm), Y79 and Weri-Rb1 cell lines were compared to normal cadaveric human retina (n = 2). On average of 7.56±1.8, 3.3±0.3 and 5.3±0.5 fold were expressed higher in RB tumors, Y79 and Weri-Rb1 respectively (Figure 1B) using β-2 microglobulin for normalization in relative quantitative PCR. Tiam1 protein levels were higher in expression compared to the normal retina on Western blot. Tiam1 protein bands were observed at ∼180 KDa, a loading control β-actin was detected at ∼42 KDa in Western blot (Figure 1C). The protein bands intensity were represented as graph in Figure 1D.
RNA interference of Tiam1 in RB cell lines
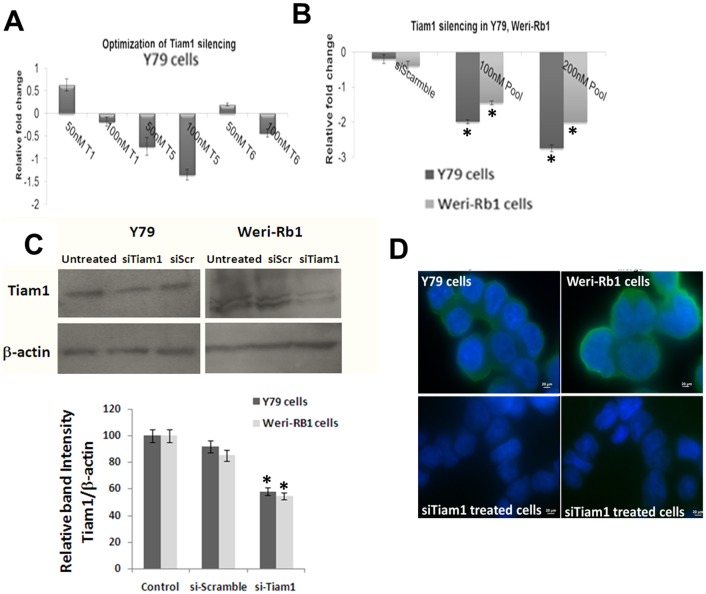
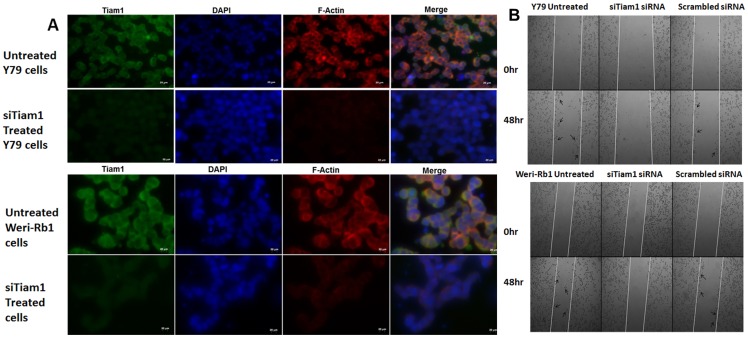
To study the cellular events mediated by Tiam1 in RB, Tiam1 knockdown is performed transiently in Y79 cells with three different siRNA sequences (T1, T5 and T6) as mentioned in methods. After 48 h of transfection, the silencing efficiency of each siRNA duplexes was determined by Real-time PCR (Figure 2A). Individual siRNA duplexes did not show significant down-regulation of Tiam1 in Y79. As per Parsons et al. (2009) the silencing efficiency was increased when all the three siRNA sequences were pooled and transfected [29]. 200 nM of siRNA pools showed –2.75 and −2.0 fold down regulation in Y79 and Weri-Rb1 respectively (Figure 2B). This was further confirmed at protein level by Western blotting (Figure 2C). The intensity of protein bands were measured using ImageJ software and percentage of Tiam1 expression upon siRNA treatment was calculated and represented as graph. In general, Tiam1 is localized in both plasma membrane and cytoplasm of untransfected cells but the expression level on plasma membrane was drastically reduced upon Tiam1 silencing in Y79 and Weri-Rb1 cells (Figure 2D).
Figure 2. Knockdown of Tiam1 in Y79 and Weri-Rb1 cell lines using RNA interference.
A. Tiam1 silencing in Y79 cells using three siRNA sequences (T1, T5 & T6) targeting different regions of the mRNA at 50 nM and 100 nM concentrations. B. Down-regulation of Tiam1 in Y79 and Weri-Rb1 cells compared to scrambled siRNA mediated by transfection of 100 nM and 200 nM of pooled siRNA. The statistical analysis was calculated using students unpaired t-test and p<0.05 is indicated as asterisk. C. Western blot analysis of Tiam1 post silencing in Y79 and Weri-Rb1 cell lines. β-actin showing a band at 42 KDa is used to normalize within sample and between siRNA transfected and untransfected cells, on the right is the densitometry analysis of western blot showing the relative band intensity of Tiam1 expression of siRNA transfected cells compared to scrambled siRNA transfected cells. The statistical analysis was calculated using students unpaired t-test and p<0.05 is indicated as asterisk. D. Immunofluorescence images of Tiam1 silenced Y79 and Weri-Rb1 cells showing the reduction of Tiam1 expression on plasma membrane compared to untransfected cells showing high level of Tiam1 expression on plasma membrane. The images were taken at ten fields under 100X oil immersion objective. Scale bar: 20 μm. Tiam1 is shown as green and nucleus counterstained with DAPI fluoresces blue in color.
Tiam1 regulates apoptosis and viability in RB cells
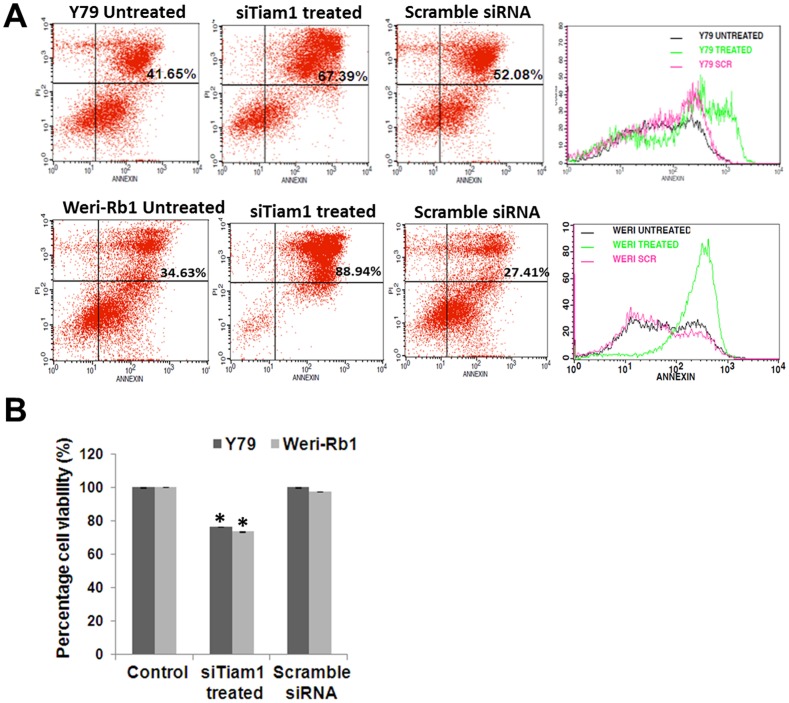
Further to understand the functional relevance behind the expression of Tiam1 in cellular activities such as apoptosis and viability, studies were carried out in RB cell lines, Y79 and Weri-Rb1 in the presence and absence of Tiam1. To elucidate apoptotic effects upon silencing of Tiam1 Annexin-V assay was performed. Results showed 15% and 50% of late apoptotic cells in Y79 and Weri-Rb1 cells respectively upon siTiam1 (Figure 3A). Upon silencing with Tiam1, the cellular metabolic activity of Y79 and Weri Rb1 decreased by 25% in MTT assay (Figure 3B).
Figure 3. Analysis of Apoptosis and viability of Tiam1 silenced Y79 and Weri-Rb1 cells.
A. invitro cell apoptosis assay showing the difference in mean fluorescence intensity between Tiam1 siRNA treated and Scrambled siRNA treated Y79, Weri-Rb1 cells. On the right panel, overlay graph showing the Annexin V expression between the samples. B. MTT assay showing the significant reduction in cell viability in Tiam1 silenced RB cell lines. Error bar represents the standard deviation of triplicates value and the statistical analysis was calculated using students unpaired t-test and p<0.05 is indicated as asterisk.
siRNA mediated Tiam1 silencing results in de-regulation of gene expression in Y79 cells
cDNA microarray was performed to analyze the genes regulated upon Tiam1 knock down using Y79 RB cell line. Raw data files were normalized using GeneSpring GX v 12.0. A total of 790 transcripts were observed to be differentially expressed in Tiam1 silenced cells above 1.0 fold (p<0.05) of which 302 genes were up-regulated and 488 genes were down-regulated. Hierarchical clustering of differentially regulated genes was done using Pearson uncentered distance matrix and average linkage rule to establish gene clusters that differentiate the two groups of samples (Figure 4A). Biological analysis of differentially expressed genes was done for Gene Ontology and Pathways using DAVID tool (http://david.abcc.ncifcrf.gov/). Statistically significant ontologies and pathways were filtered based on p-Value <0.05 (Obtained using Fischer Exact Test) with Benjamini Hocheberg FDR correction (Figure S1). The data obtained from microarray analysis was deposited in NCBI's Gene Expression Omnibus (GEO45130) as per MIAME guidelines. The significantly de-regulated genes in response to Tiam1 silencing are listed in Table S1.
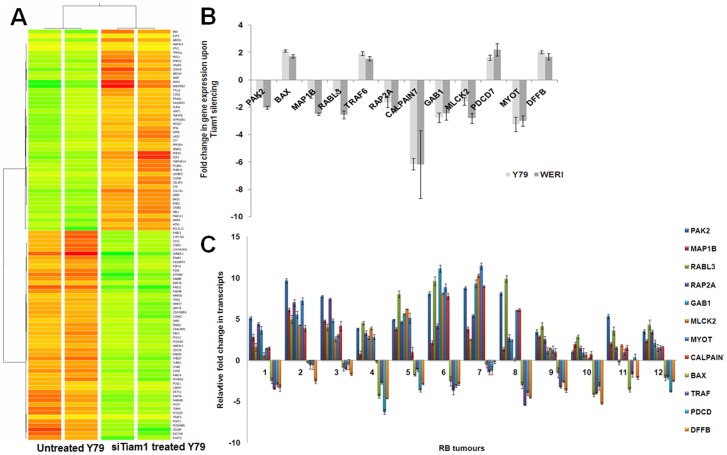
Figure 4. cDNA microarray in Tiam1 silenced Y79 and validation of de-regulated genes in RB cell lines & tumors.
A. Whole genome microarray of Tiam1 silenced Y79 cells was performed using Human-Ht12 beadChip ver. 3 platform. Hierarchical Cluster represents the expression profile of de-regulated genes upon Tiam1 silencing in Y79 cells, compared to untransfected cells. B. Real-time PCR results showing the mRNA expression of selected genes from microarray analysis in Tiam1 silenced Y79 and Weri-Rb1 cells C. Relative fold change in the mRNA expression of selected panel of genes verified in primary retinoblastoma showing the negative correlation to that of Tiam1 silenced Y79 and Weri-Rb1 retinoblastoma cells. Error bar represents the standard deviation of triplicates value.
Significantly down-regulated genes in Tiam1 silenced Y79 cells
Since Tiam1 acts as GEF, silencing of Tiam1 led to down- regulation of most of the RAS (rat sarcoma) oncogene family of small GTPases such as RAB8B, RAP2A, RHOT2, RAB3D, RAB14, RAB40B, and RABL3, actin cytoskeleton genes- Homo sapiens p21 (CDKN1A)-activated kinase 2 (PAK2), Homo sapiens CDC42 binding protein kinase gamma (CDC42BPG), Homo sapiens CD2-associated protein (CD2AP), Homo sapiens fibroblast growth factor 16 (FGF16), Homo sapiens actin-like protein (FKSG30), Homo sapiens microtubule-associated protein 1B (MAP1B)., focal adhesion genes- Homo sapiens tubulin, delta 1 (TUBD1), Homo sapiens chondroadherin (CHAD), Homo sapiens myotilin (MYOT), Homo sapiens myosin light chain kinase 2 (MYLK2), Homo sapiens PDGFA associated protein 1 (PDAP1), Homo sapiens C-terminal binding protein 2 (CTBP2), Homo sapiens CREB regulated transcription coactivator 1 (CRTC1), Homo sapiens matrix metallopeptidase 27 (MMP27), Homo sapiens matrix metallopeptidase 28 (MMP28), Homo sapiens GRB2-associated binding protein 1 (GAB1), Homo sapiens calpain 7 (CAPN7).
Significantly up-regulated genes in Tiam1 silenced Y79 cells
Tiam1 knock down in Y79 cells resulted in up-regulation of apoptotic genes- Homo sapiens BCL2-associated X protein (BAX), Homo sapiens programmed cell death 7 (PDCD7), Homo sapiens DNA fragmentation factor (DFFB), Homo sapiens TNF receptor-associated factor 6 (TRAF6), Homo sapiens BCL2-like 12 (BCL2L12), GTP binding genes- Homo sapiens Rho family GTPase 2 (RND2), Homo sapiens RAS guanyl releasing protein 2 (RASGRP2), (multiple drug resistance genes) Homo sapiens ATP-binding cassette, sub-family G member 4 (ABCG4), Homo sapiens ATP-binding cassette, sub-family D member 1 (ABCD1), Homo sapiens cAMP responsive element binding protein 1 (CREB1), and Homo sapiens mitogen-activated protein kinase kinase kinase 4 (MAP3K4), Homo sapiens collagen, type VII, alpha 1 (COL7A1), Homo sapiens suppression of tumorigenicity 5 (ST5), Homo sapiens suppression of tumorigenicity 7 (ST7), Homo sapiens tumor protein p63 regulated 1-like (TPRG1L), Homo sapiens claudin 6 (CLDN6), Homo sapiens cadherin 15 (CDH15).
Validation of de-regulated genes by qPCR in Tiam1 silenced RB cell lines and primary RB tumors
From the de-regulated genes list, a panel of genes namely PAK2, MAP1B, RABL3, RAP2A, GAB1, MLCK2, MYOT, CALPAIN7, BAX, PDCD, TRAF6, DFFB were selected for the confirmation of microarray analysis by quantitative PCR in Tiam1 silenced Y79 and Weri-Rb1 cells (Figure 4B). The list of primer sequences used for the SYBR-green based qPCR is given in Table 1. Genes involved in actin cytoskeleton were down-regulated where as apoptotic genes were up-regulated in post Tiam1 silenced Y79 and Weri-Rb1 cells. The mRNA expressions of these genes in both RB cell lines were consistent with microarray analysis. Similarly qRT-PCR was done to evaluate the correlation of the validated genes expression in primary RB tumors (Figure 4C). mRNA expression of these selected genes were negatively correlate with primary RB tumors. The average fold expression of 12 genes in 12 tumors normalized to two normal retina were, PAK2 (5.78), MAP1B (2.88), RABL3 (5.00), RAP2A (3.99), GAB1 (4.10), MLCK2 (3.47), MYOT (4.15), CALPAIN7 (3.17), BAX (−2.25), TRAF6 (−2.53), PDCD7 (−2.60), DFFB (−3.00). The list of the primary tumors used and its clinic-pathological descriptions, showing the expression levels of validated genes are given in Table 2. Tumors with CI<3 mm showed lesser extent of changes in gene expression compared to tumors with CI>3 mm (tumor 2, 3, 5, 6, 7). qPCR results were shown in Figure 4 & CI status were shown in Table 2. The expression levels of Tiam1 is also directly correlating to the changes in gene expression of validated targets in RB tumor.
Table 1. Primer sequences for the selected panel of validated genes.
| GENE | PRIMER SEQUENCES | |
| RAP2A | FP: AGA TCA TCC GCG TGA AGC | RP: CCC CAC TCT TCA GCA AGG |
| BAX | FP: TGG AGCTGCAGAGGATGATTG | RP: GAAGTTGCCGTCAGAAAACATG |
| Rabl3 | FP: TTGGGAGACTCAGGTGTTGGGAAA | RP: CAGTTGGCACCAAATCCCTGTTGA |
| PAK2 | FP: GAATGGAAGGATCTGTTAAGCTCACT | RP: GCCATAAGCTTTCCGTGTAACC |
| MAP1B | FP: AAAGTGTCCAGGGTGGCTTC | RP: CTCCTGGTACCATTCCCTCA |
| TRAF6 | FP: TGGCATTACGAGAAGCAGTG | RP: GTTCCATCTTGTGCAAACAACC |
| DFFB | FP: GGCCTGCTTTTTACCTCAGA | RP: CGTTTCCGCACAGGCTGCTT |
| MLCK2 | FP: GAGCTGAGGACCGGGAAT | RP: AGGTACAGACTGCCCCAAAC |
| CALPAIN7 | FP: ATCTGGAAAGAGTTCAAGCT | RP: GCACGCTCTAAGACCAACAG |
| PDCD7 | FP: TTGACCCAGGCTGCCTAT | RP: AATCTCCTGCTCGCGTTC |
| MYOT | FP: CGACTGCAAGTTCCTACATCAC | RP: TGAATGAAACGTGGTGGGTA |
| GAB1 | FP: ACCTCAAGCCAGACAGAAAAGT | RP: TCGAGCAAAACTCCTAGTGATG |
Table 2. Clinicopathological features of primary Retinoblastoma tumors and gene expression profile by QRT-PCR.
| S.No. | Age/ Sex | Clinicopathological descriptions | PAK2 | MAP1B | RABL3 | RAP2A | GAB1 | MLCK2 | MYOT | CALPAIN7 | BAX | TRAF6 | PDCD7 | DFFB |
| 1 | 2/M | RB, PD, Focal CI<3mm, Pre laminar& Post laminar invasion of ON. | UR | UR | UR | UR | UR | NS | UR | UR | DN | DN | DN | DN |
| 2 | 2/M | RB, UD, CI>3mm, Tumor cells invading in anterior border of sclera. | UR | UR | UR | UR | UR | UR | UR | UR | NS | NS | NS | DN |
| 3 | 2/F | RB, PD, CI>3mm, Pre laminar & Post laminar invasion of tumor. | UR | UR | UR | UR | UR | UR | UR | UR | NS | DN | NS | DN |
| 4 | 2/F | RB, WD, CI<3mm, No invasion of ON. | UR | NS | UR | UR | UR | UR | UR | NS | DN | DN | DN | DN |
| 5 | 6/M | RB, CI>3mm, Tumor cells touching the anterior border of the sclera. | UR | UR | UR | UR | UR | UR | UR | UR | DN | DN | DN | DN |
| 6 | 3/F | RB, MD, CI>3mm, Pre laminar& Post laminar invasion of ON. | UR | UR | UR | UR | UR | UR | UR | UR | DN | DN | DN | DN |
| 7 | 4/M | RB, PD, CI>3mm, Pre laminar& Post laminar invasion, Tumor invasion into anterior, middle& posterior border of sclera. | UR | UR | UR | UR | UR | UR | UR | UR | NS | DN | DN | NS |
| 8 | 1/F | RB, WD, CI<3mm, No invasion of ON | UR | UR | UR | UR | UR | NS | UR | UR | DN | DN | DN | DN |
| 9 | 3/F | RB, PD, No CI, Pre laminar &Post laminar invasion of ON. | UR | UR | UR | UR | UR | UR | UR | UR | DN | DN | DN | DN |
| 10 | 2/M | RB, PD, NoCI, Pre laminar invasion of ON. | UR | UR | UR | UR | NS | NS | NS | NS | DN | DN | DN | DN |
| 11 | 13mon/ M | RB, WD, NoCI, No invasion of ON | UR | UR | UR | UR | NS | UR | NS | UR | DN | DN | NS | DN |
| 12 | 11/F | RB, MD, CI<3mm | UR | UR | UR | UR | UR | UR | UR | UR | DN | DN | DN | DN |
F: Female; M: Male; RB: Retinoblastoma; PD: Poorly Differentiated; MD: Moderately Differentiated; WD: Well Differentiated; UD: Un-Differentiated; CI: Choroid Invasion; ON: Optic Nerve; UR: Up-regulation above 1 fold change (Log 2 ratio); DR: Down-regulation above 1 fold change (Log2 ratio); NS: Not Significant fold change.
Tiam1 regulates actin polymerization, cell migration and invasion in RB cell lines
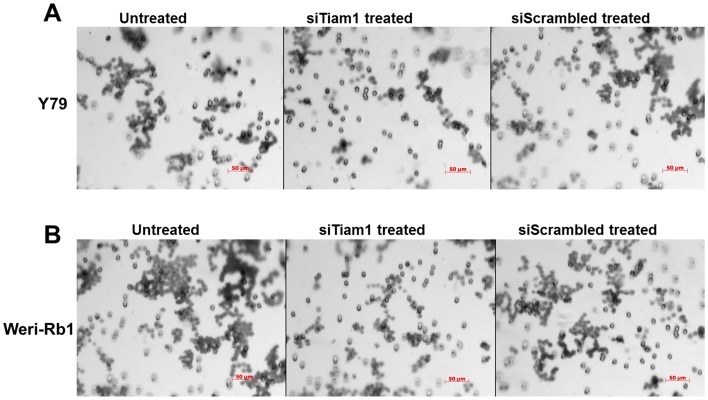
To investigate the involvement of Tiam1 in actin cytoskeleton regulation, F-actin staining was performed using phalloidin in Tiam1 silenced Y79 and Weri-Rb1 cells (Figure 5A). As mentioned above, Tiam1 was co-localized with actin at cell junctions. In case of Tiam1 knockdown, the cells exhibited lesser extent of actin polymerization at the cellular junctions and actin co-localization. Thus the results show that Tiam1 is essential for actin re-organisation in RB cells. Additionally, the function of Tiam1 in cell migration was assessed by wound healing assay (Figure 5B). Tiam1 silenced RB cell lines showed impairment of cell migration, compared to untransfected and scrambled siRNA transfected RB cells. Silencing of Tiam1 in Weri-Rb1 had resulted in lesser number of cells invaded across the matrigel coated invasion chamber. This indicated the decrease in invasion potential depends on Tiam1 expression as it is not observed in the scramble siRNA transfected cells (Figure 6).
Figure 5. F-actin staining and invasion of Tiam1 deficient RB cell lines.
A. Tiam1 silenced Y79 cells and Weri-Rb1 cells were fixed, immunofluorescently labeled for Tiam1, nucleus stained with DAPI, stained with phalloidin and images were taken at 40X in ten fields. Bar represents 20 μm. B. Phase contrast microscope images of wound healing assay showing the cell migration pattern in Tiam1 deficient retinoblastoma cell lines at 0 hr and 48 hrs post silencing. Tiam1 knockdown cells were unable to migrate whereas the untransfected and control cells showed increased migration, migrated cells were indicated with arrows. The images were acquired using AxioObserver microscope at 5× objective with 1× optovar.
Figure 6. Matrigel invasion assay of Tiam1 deficient RB cell lines.
A. Y79 panel showing the invasion of untransfected, siTiam1 treated and scrambled siRNA treated cells. B. Weri-Rb1 cells. The matrigel invasion chambers post invasion was stained with crystal violet and images acquired at 20× objective. The experimental results were representative of triplicate result repeated twice.
Plasma membrane localization of Tiam1 is mediated by N-terminal PH domain
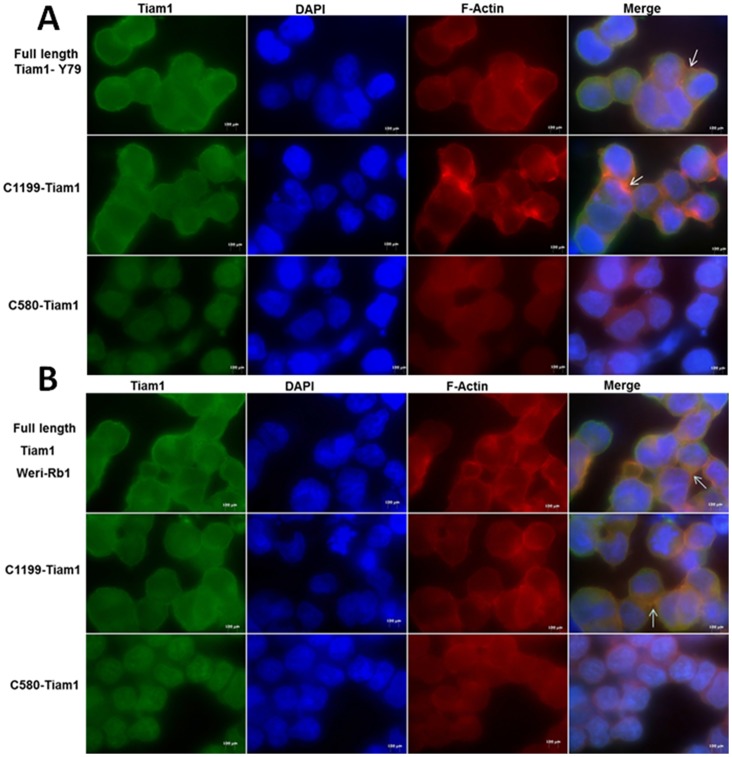
Since Tiam1 localizes along with F-actin and controls the actin cytoskeleton, we investigated which domain of the protein regulates the localization Tiam1 on plasma membrane in RB. RB cell lines (Y79 and Weri-Rb1) transfected with full length and C1199 Tiam1 showed the membrane localization and induced the membrane ruffling (Figure 7). In contrast, C580 Tiam1 was localized to the nucleus and failed to induce membrane ruffling.
Figure 7. Localization of Full length Tiam1, C1199 Tiam1 and C580 Tiam1 in RB cell lines.
A) Immunofluorescent images of Y79 cells, B) Weri-Rb1 cells transfected with Full length Tiam1, C1199 Tiam1 and C580 Tiam1 plasmids tagged with HA. Tiam1 and F-actin were stained as mentioned in the materials and methods. Full length Tiam1 and C1199 Tiam1 but not C580 Tiam1 were localized to plasma membrane. Unlike Full length Tiam1 and C1199 Tiam1, C580 Tiam1 could not induce membrane ruffling. Representative images were taken from 10 independent fields using AxioObserver fluorescent microscope at 100× and arrows indicate the membrane ruffling. Scale bar: 20 μm.
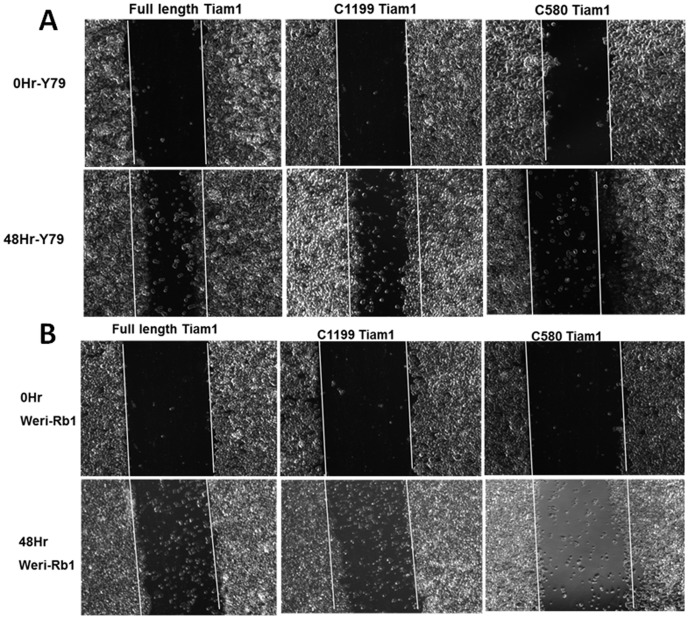
N-terminal PH domain regulates retinoblastoma cell motility
We observed that N-terminal PH domain, but not C-terminal PH domain modulates the localization of Tiam1 and induces membrane ruffling in RB cells. Further we determined the association of membrane localization of Tiam1 and cell migration in Y79, Weri-Rb1 cells. To elucidate this, wound healing assay was performed in Y79 and Weri-Rb1 cells. The cells transfected with full length Tiam1 and C1199 Tiam1 were showing more cell migration towards the wound, whereas C580 Tiam1 transfected cells showed delayed cell migration into the wound in both cell lines (Figure 8).
Figure 8. N-terminal PH domain maintains cell motility in retinoblastoma cells.
Phase contrast images showing the migration of Y79 and Weri-Rb1 cells transfected with Full length Tiam1, C1199 Tiam1 and C580 Tiam1 in wound-healing assay. White line indicates the original wound edge which made by a pipette tip. The images were taken from 10 different locations. Cells transfected with full length and C1199 Tiam1 showing significant increase in cell migration rate compared to the cells transfected with C580 Tiam1. The Images were captured at 5× objective in AxioObserver microscope.
Discussion
Though Tiam1 has been shown to play a crucial role in actin cytoskeleton, cell migration and invasion, various intracellular pathways are involved in activation of upstream and downstream signaling of Tiam1 (Figure 9) [9], [10], [13], [27]. In particular, Tiam1 is required for activation of Rac1 and cdc42 to produce specific actin rich structures like membrane ruffling, lamellipodia, filopodia [30], [31], [32], [33], [34], [35] and for neurite outgrowth [36]. Addition to that, Tiam1 interacts with CADM1, Ephrin and Arp2/3 to initiate Rac1 mediated actin cytoskeleton remodeling [37], [38], [39]. Hence in the current study, we analysed the importance of Tiam1 in RB cells using short interfering RNA (siRNA) mediated knockdown studies.
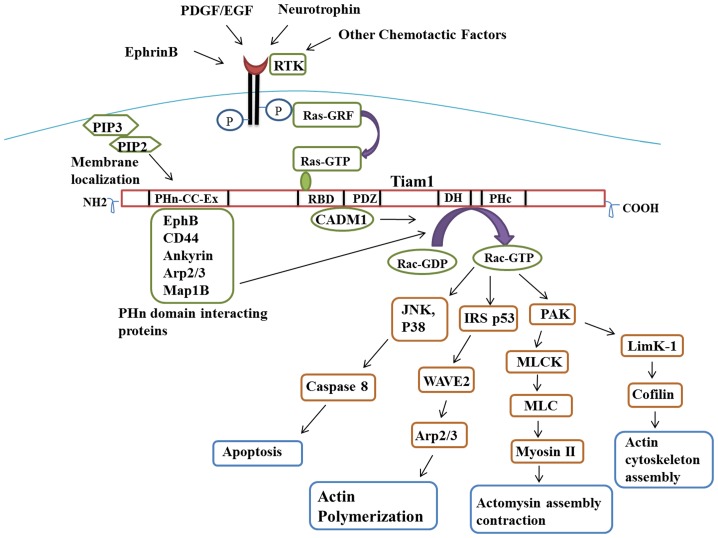
Figure 9. Tiam1 mediated signaling pathway.
Schematic representation showing the upstream and downstream effects of Tiam1 in intracellular pathways. External stimulants phosphorylate the receptor tyrosine kinase which inturn activates Ras-GTP, binds to RBD domain of Tiam1. Interaction of EphB, CD44 and Ankyrin Proteins with PHn domain of Tiam1 and CADM1 with PDZ domain leads to the activation of Rac-GTP at DH domain of Tiam1. The activated Rac-GTP involves in various signaling pathways, mainly on actin cytoskeleton. The binding of PHn domain to phosphoinositide facilitates the membrane localization of Tiam1. PDGF: Platelet-derived growth factor; EGF: Epidermal growth factor; RTK: Receptor tyrosine kinase; PIP3: Phosphatidyl inositol triphosphate; PIP2: Phosphatidyl inositol diphosphate; PHn: N-terminal PH domain; RBD: Ras binding domain; PDZ: PSD-95/DglA/ZO-1 domain; DH: Dgl homology domain; PHc: C-terminal PH domain; JNK: Jun N-terminal kinase; PAK: p21-activated kinase.
Silencing of Tiam1 in RB cell lines followed by cDNA microarray showed various pathways and genes altered, predominantly genes related to MAPK pathway, small GTPase, apoptosis and cell migration. The impairment of cell migration in Tiam1 silenced Y79 and Weri-Rb1 cells was proved by Wound healing and matrigel invasion assay. This correlates with the microarray analysis where the actin cytoskeleton genes were down-regulated in Tiam1 silenced Y79 cells resulting in lesser cellular migration potential (Figure 5). One of the down-regulated actin cytoskeleton gene in our microarray analysis is MAP1B (Microtubule-associated protein 1B) which is needed for axonal development, reported to be involved in neurite growth, neuron migration and metastasis [40], [41], [42]. MAP1B is found to interact with Tiam1 thereby activating Rac1 and cdc42 and further inhibiting RhoA activity which leads to actin polymerization and axonal elongation [43], [44]. MAP1B deficient cells exhibit a decreased cell migration and axonal development [45], [46]. The other mechanism might be PAK mediated activation of MyosinII, a protein involved in stress fiber formation and contraction [47]. The phosphorylation of myosinII light chain (MLC) by myosinII light chain kinase (MLCK) regulates actin-myosin II interaction [48]. We observed that PAK2 and MLCK were down-regulated in Tiam1 silenced retinoblastoma Y79 cells. Moreover, small GTPase subfamily Rab-like 3 (Rabl3) and actin-binding protein Myotilin, promote motility, tumor cell survival [49]. The down-regulation of Rabl3 and myotilin might as well attribute to the suppression of cell motility in Tiam1 silenced cells. The expression level of these genes when validated in primary RB tumors showed differential expression correlating with their CI status. CI represents the invasion potential of the given tumor during the enucleation, which may or may not have undergone chemotherapeutic treatment. The tumors with CI>3 mm compared to CI<3 mm cases showed increased expression of pro-survival genes and decreased expression of apoptotic genes.
From our Annexin-V assay results, a remarkable increase in apoptosis was observed. The mechanism of apoptotic induction might be due the up-regulation of pro-apoptotic gene BAX (Bcl-2 associated X), PDCD7 (Programmed cell death protein), TRAF6 (Tumor necrosis factor receptor-associated factor 6) and DFFB (DNA fragmentation factor subunit beta) upon Tiam1 knockdown [50], [51], [52], [53], [54]. Similar to our results, apoptotic cell death has been observed earlier upon Tiam1 silencing [55].
Since endogenous Tiam1 is localized in both plasma membrane and cytoplasm, we were interested to find out which domain of the Tiam1 protein regulates the localization intracellularly in RB cells. We elucidated that N-terminal PH domain of Tiam1 mediates the membrane localization and invasion but not the C-terminal PH domain and targeting the N-terminal PH domain of Tiam1 affects the cell migration and invasion. Earlier studies showed that localization of Tiam1 to plasma membrane requires N-terminal PH domain. Also the membrane localization of Tiam1 is required for the activation of the c-Jun NH2-terminal kinase (JNK) thus leading to successful Rac mediated signaling [13]. N-terminal PH domain of Tiam1 has affinity towards phosphoinositides and its interaction with transmembrane domain accelerates the membrane localization. For the first time our data has additionally elucidated the importance of the N-terminal region with respect to the migration as it is directly correlating with actin cytoskeleton modulation in RB. From our findings, we suggest that Tiam1 can be utilized as a potential target for RB therapy. In future, docking studies to find potent inhibitors to RBD binding or alternatively aptamers targeting the N-terminal PH domain of Tiam1 need to be isolated, which have better application in vivo for drug delivery purposes.
Supporting Information
Pathways and gene ontology altered by Tiam1. A. Pie chart represents significantly de-regulated pathways in response to Tiam1 silencing. B. Graphical representation of significantly de-regulated gene ontology.
(TIF)
Deregulated genes in Tiam1 silenced Y79 cells. List of genes shortlisted and used for Hierarchial clustering and further pathway and gene ontology analysis.
(DOC)
Acknowledgments
We thank Mr. Madavan Vasudevan, Bionivid technologies Pvt. Ltd for microarray analysis, Dr. Vikas Khetan for providing us with primary retinoblastoma patient samples, John G Collard, Netherlands Cancer Institute for providing the Tiam1 plasmid constructs. Deakin University is acknowledged for providing scholarship to Ms. Nithya Subramanian under Graduate student program (code: S911, Student No. 211640938).
Funding Statement
This work was supported by the Indian Council for Medical Research, Grant No. ICMR/5/13/77/2008-NCDIII. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sengupta S, Krishnakumar S, Sharma T, Gopal L, Khetan V (2013) Histopathology of retinoblastoma: does standardization make a difference in reporting? Pediatr Blood Cancer 60: 336–337. [DOI] [PubMed] [Google Scholar]
- 2. Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, et al. (1994) Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77: 537–549. [DOI] [PubMed] [Google Scholar]
- 3. Habets GG, van der Kammen RA, Stam JC, Michiels F, Collard JG (1995) Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene 10: 1371–1376. [PubMed] [Google Scholar]
- 4. Mertens AE, Roovers RC, Collard JG (2003) Regulation of Tiam1-Rac signalling. FEBS Lett 546: 11–16. [DOI] [PubMed] [Google Scholar]
- 5. Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG (1995) A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375: 338–340. [DOI] [PubMed] [Google Scholar]
- 6. Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, et al. (1997) Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278: 1464–1466. [DOI] [PubMed] [Google Scholar]
- 7. Adams HC 3rd, Chen R, Liu Z, Whitehead IP (2010) Regulation of breast cancer cell motility by T-cell lymphoma invasion and metastasis-inducing protein. Breast Cancer Res 12: R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu K, Rajagopal S, Klebba I, Dong S, Ji Y, et al. (2010) The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene 29: 6533–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connolly BA, Rice J, Feig LA, Buchsbaum RJ (2005) Tiam1-IRSp53 complex formation directs specificity of rac-mediated actin cytoskeleton regulation. Mol Cell Biol 25: 4602–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, et al. (1998) Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol 143: 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180. [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Zhang Q, Zhang Y, Wang S, Ding Y (2006) Lentivirus-mediated silencing of Tiam1 gene influences multiple functions of a human colorectal cancer cell line. Neoplasia 8: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls-Van Stalle L, et al. (1997) Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J Cell Biol 137: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Leeuwen FN, van der Kammen RA, Habets GG, Collard JG (1995) Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene 11: 2215–2221. [PubMed] [Google Scholar]
- 15. Engers R, Zwaka TP, Gohr L, Weber A, Gerharz CD, et al. (2000) Tiam1 mutations in human renal-cell carcinomas. Int J Cancer 88: 369–376. [DOI] [PubMed] [Google Scholar]
- 16. Qi Y, Huang B, Yu L, Wang Q, Lan G, et al. (2009) Prognostic value of Tiam1 and Rac1 overexpression in nasopharyngeal carcinoma. ORL J Otorhinolaryngol Relat Spec 71: 163–171. [DOI] [PubMed] [Google Scholar]
- 17. Yang W, Lv S, Liu X, Liu H, Hu F (2010) Up-regulation of Tiam1 and Rac1 correlates with poor prognosis in hepatocellular carcinoma. Jpn J Clin Oncol 40: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 18. Adithi M, Venkatesan N, Kandalam M, Biswas J, Krishnakumar S (2006) Expressions of Rac1, Tiam1 and Cdc42 in retinoblastoma. Exp Eye Res 83: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 19. Zhao L, Liu Y, Sun X, He M, Ding Y (2011) Overexpression of T lymphoma invasion and metastasis 1 predict renal cell carcinoma metastasis and overall patient survival. J Cancer Res Clin Oncol 137: 393–398. [DOI] [PubMed] [Google Scholar]
- 20. Zhong D, Li Y, Peng Q, Zhou J, Zhou Q, et al. (2009) Expression of Tiam1 and VEGF-C correlates with lymphangiogenesis in human colorectal carcinoma. Cancer Biol Ther 8: 689–695. [DOI] [PubMed] [Google Scholar]
- 21. Liu L, Wu DH, Ding YQ (2005) Tiam1 gene expression and its significance in colorectal carcinoma. World J Gastroenterol 11: 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engers R, Mueller M, Walter A, Collard JG, Willers R, et al. (2006) Prognostic relevance of Tiam1 protein expression in prostate carcinomas. Br J Cancer 95: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collard JG, Habets GG, Michiels F, Stam J, van der Kammen RA, et al. (1996) Role of Tiam 1 in Rac-mediated signal transduction pathways. Curr Top Microbiol Immunol 213 (Pt 2): 253–265. [DOI] [PubMed] [Google Scholar]
- 24. Crompton AM, Foley LH, Wood A, Roscoe W, Stokoe D, et al. (2000) Regulation of Tiam1 nucleotide exchange activity by pleckstrin domain binding ligands. J Biol Chem 275: 25751–25759. [DOI] [PubMed] [Google Scholar]
- 25. Terawaki S, Kitano K, Mori T, Zhai Y, Higuchi Y, et al. (2010) The PHCCEx domain of Tiam1/2 is a novel protein- and membrane-binding module. EMBO J 29: 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HE, et al. (1997) Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem 272: 28447–28454. [DOI] [PubMed] [Google Scholar]
- 27. Ceccarelli DF, Blasutig IM, Goudreault M, Li Z, Ruston J, et al. (2007) Non-canonical interaction of phosphoinositides with pleckstrin homology domains of Tiam1 and ArhGAP9. J Biol Chem 282: 13864–13874. [DOI] [PubMed] [Google Scholar]
- 28. Fleming IN, Batty IH, Prescott AR, Gray A, Kular GS, et al. (2004) Inositol phospholipids regulate the guanine-nucleotide-exchange factor Tiam1 by facilitating its binding to the plasma membrane and regulating GDP/GTP exchange on Rac1. Biochem J 382: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsons BD, Schindler A, Evans DH, Foley E (2009) A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS One 4: e8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demarco RS, Struckhoff EC, Lundquist EA (2012) The Rac GTP exchange factor TIAM-1 acts with CDC-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet 8: e1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamauchi J, Miyamoto Y, Tanoue A, Shooter EM, Chan JR (2005) Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc Natl Acad Sci U S A 102: 14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bourguignon LY, Zhu H, Shao L, Chen YW (2000) Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic breast tumor cell invasion and migration. J Cell Biol 150: 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bourguignon LY, Zhu H, Shao L, Chen YW (2000) CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J Biol Chem 275: 1829–1838. [DOI] [PubMed] [Google Scholar]
- 34. Matsuo N, Terao M, Nabeshima Y, Hoshino M (2003) Roles of STEF/Tiam1, guanine nucleotide exchange factors for Rac1, in regulation of growth cone morphology. Mol Cell Neurosci 24: 69–81. [DOI] [PubMed] [Google Scholar]
- 35. Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A (2009) Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol 77: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shirazi Fard S, Kele J, Vilar M, Paratcha G, Ledda F (2010) Tiam1 as a signaling mediator of nerve growth factor-dependent neurite outgrowth. PLoS One 5: e9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masuda M, Maruyama T, Ohta T, Ito A, Hayashi T, et al. (2010) CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells. J Biol Chem 285: 15511–15522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, et al. (2007) The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A 104: 7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka M, Ohashi R, Nakamura R, Shinmura K, Kamo T, et al. (2004) Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J 23: 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malliri A, van Es S, Huveneers S, Collard JG (2004) The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem 279: 30092–30098. [DOI] [PubMed] [Google Scholar]
- 41. Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, et al. (2006) The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J Biol Chem 281: 543–548. [DOI] [PubMed] [Google Scholar]
- 42. Cajanek L, Ganji RS, Henriques-Oliveira C, Theofilopoulos S, Konik P, et al. (2013) Tiam1 regulates the Wnt/Dvl/Rac1 signaling pathway and the differentiation of midbrain dopaminergic neurons. Mol Cell Biol 33: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buongiorno P, Pethe VV, Charames GS, Esufali S, Bapat B (2008) Rac1 GTPase and the Rac1 exchange factor Tiam1 associate with Wnt-responsive promoters to enhance beta-catenin/TCF-dependent transcription in colorectal cancer cells. Mol Cancer 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ten Klooster JP, Evers EE, Janssen L, Machesky LM, Michiels F, et al. (2006) Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem J 397: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tymanskyj SR, Scales TM, Gordon-Weeks PR (2012) MAP1B enhances microtubule assembly rates and axon extension rates in developing neurons. Mol Cell Neurosci 49: 110–119. [DOI] [PubMed] [Google Scholar]
- 46. Gordon-Weeks PR, Fischer I (2000) MAP1B expression and microtubule stability in growing and regenerating axons. Microsc Res Tech 48: 63–74. [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez-Billault C, Del Rio JA, Urena JM, Jimenez-Mateos EM, Barallobre MJ, et al. (2005) A role of MAP1B in Reelin-dependent neuronal migration. Cereb Cortex 15: 1134–1145. [DOI] [PubMed] [Google Scholar]
- 48. Cueille N, Blanc CT, Popa-Nita S, Kasas S, Catsicas S, et al. (2007) Characterization of MAP1B heavy chain interaction with actin. Brain Res Bull 71: 610–618. [DOI] [PubMed] [Google Scholar]
- 49. Montenegro-Venegas C, Tortosa E, Rosso S, Peretti D, Bollati F, et al. (2010) MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol Biol Cell 21: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tortosa E, Montenegro-Venegas C, Benoist M, Hartel S, Gonzalez-Billault C, et al. (2011) Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J Biol Chem 286: 40638–40648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzalez-Billault C, Avila J, Caceres A (2001) Evidence for the role of MAP1B in axon formation. Mol Biol Cell 12: 2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takei Y, Teng J, Harada A, Hirokawa N (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol 150: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakayama M, Amano M, Katsumi A, Kaneko T, Kawabata S, et al. (2005) Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells 10: 107–117. [DOI] [PubMed] [Google Scholar]
- 54. Shin DH, Chun YS, Lee KH, Shin HW, Park JW (2009) Arrest defective-1 controls tumor cell behavior by acetylating myosin light chain kinase. PLoS One 4: e7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, et al. (2000) Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem 275: 18366–18374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pathways and gene ontology altered by Tiam1. A. Pie chart represents significantly de-regulated pathways in response to Tiam1 silencing. B. Graphical representation of significantly de-regulated gene ontology.
(TIF)
Deregulated genes in Tiam1 silenced Y79 cells. List of genes shortlisted and used for Hierarchial clustering and further pathway and gene ontology analysis.
(DOC)