Figure 4.
Gas Phase Dissociation of Tryptophan Synthase
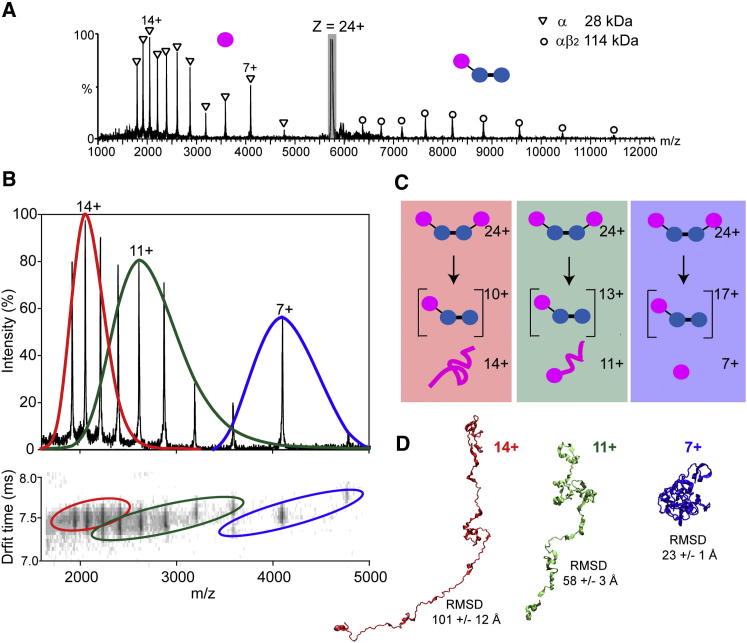
(A) MS/MS of tryptophan synthase (24+) reveals loss of α subunit.
(B) Three distinct populations of ejected α subunit are observed and confirmed via a drift time versus m/z contour plot.
(C) Schematic for the proposed parallel routes for gas phase dissociation: loss of high, intermediate, and low-charge subunits (red, green, and blue).
(D) MD simulations of tryptophan synthase (24+), over a linear temperature gradient, recapitulates the ejection of an α-monomer with different degrees of compactness. Charges assigned to the α subunit were 14+, 11+, and 7+, as determined experimentally. Residual charges were evenly distributed over the accessible basic residues on the remaining three subunits. The rmsd of the dissociated α subunit from that bound in the native structure is shown.
See also Figure S4.