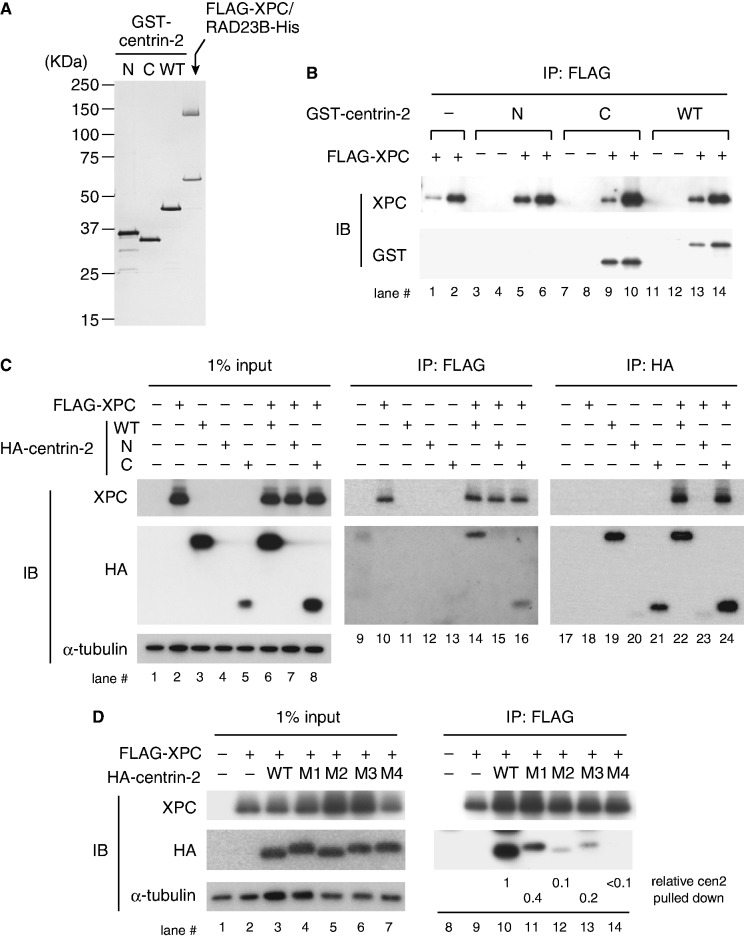
Figure 1.
The C-terminal half of centrin-2 is necessary and sufficient for interaction with XPC. (A) Purified GST-centrin-2 (WT, N or C) and the FLAG-XPC/RAD23B-His complex (0.1 µg each) were subjected to SDS–PAGE followed by silver staining. (B) Purified GST-centrin-2 (WT, N or C) or GST alone (as a negative control) was incubated with FLAG-XPC/RAD23B-His and then pulled down with anti-FLAG antibody beads. Two different amounts (4 and 8%) of the precipitated proteins were subjected to immunoblotting with the indicated antibodies. (C) FLAG-XPC and HA-centrin-2 were transiently expressed in XP4PASV cells and solubilized proteins were subjected to immunoprecipitation with anti-FLAG and anti-HA (3F10) antibodies as indicated. The precipitated proteins, along with 1% of the input extracts, were subjected to immunoblotting with anti-XPC and anti-HA (12CA5) antibodies, respectively. (D) FLAG-XPC and HA-centrin-2 point mutants (M1–M4) were transiently expressed in XP4PASV cells and subjected to immunoprecipitation with anti-FLAG antibody. The amino acids changed to alanines in each mutant are as follows: M1, F113A/L133A; M2, F113A/M145A; M3, L112A/F113A/L133A; M4. L112A/F113A/L133A/M145A. For lanes 10–14, the amounts of centrin-2 pulled down were quantified, normalized with the amounts of XPC and expressed as relative values (the value of WT centrin-2 was set as 1).