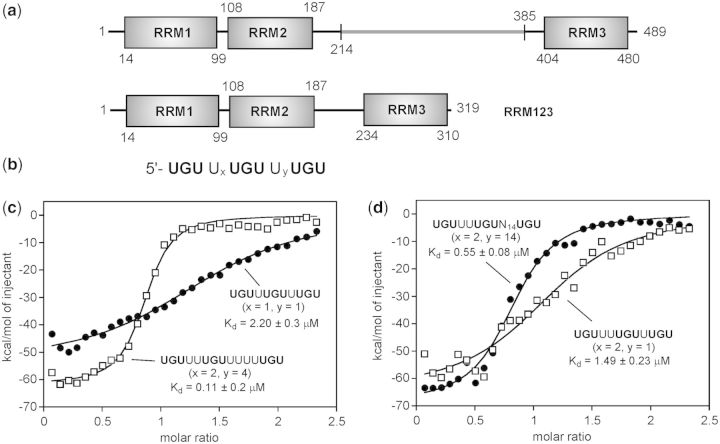
Figure 1.
Full-length sequence of CELF1 showing RRM domains and linker sequences; alignment with the Δ(215–384) mutant RRM123 (a). RNA-binding sequence for CELF1 with three UGU sites separated by Ux and Uy spacers (b). Binding curves from ITC studies of CELF1 construct RRM123 with the RNA sequences EDEN11 (x = 1, y = 1) (GRE), EDEN-2U/4U (x = 2, y = 4) (c), and for EDEN-2U/1U (x = 2, y = 1) and EDEN-2U/HL (x = 2, y = 14) (d) (see sequences in Table 1). Total heat released is plotted as a function of the molar ratio of RNA to protein, and the non-linear least squares fit to the experimental data are shown as a solid line. Dissociation constants, binding stoichiometries and enthalpies of binding are shown in Table 2.