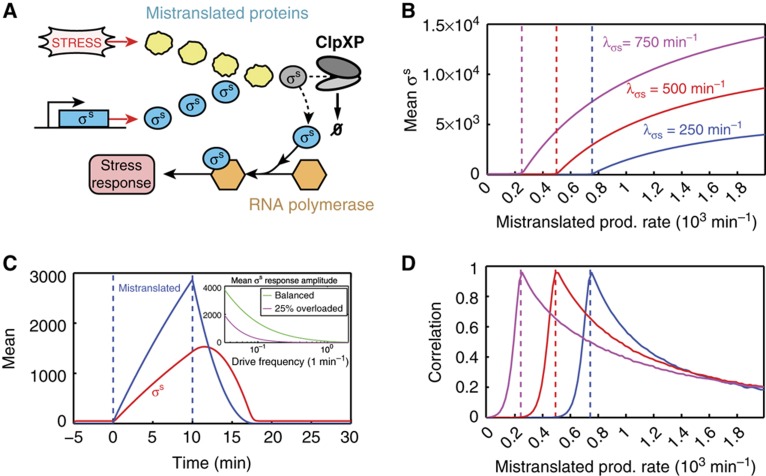
Figure 2.
The E. coli stress response network employs queueing as a signaling mechanism to ensure the most rapid response possible to adverse conditions. (A) Certain types of stress cause the accumulation of a large amount of aberrant proteins, which are targeted for rapid degradation and compete with the master stress regulator, σs, for a limited amount of ClpXP machinery. This leads to a decrease in the effective degradation rate of σs, allowing it to build up rapidly and initiate an immediate response. See the Supplementary Information for a precise definition of the model and for simulation details. (B) For a stochastic queueing model with 100 ClpXP molecules that each have processing rate μ=10 min−1, with cells dividing every 20 min, a scan of the mean steady-state level of σs with respect to the stress level (mistranslated protein production rate λm) demonstrates a very sensitive response of the system once the system has crossed the balance point (respectively colored dashed lines). Different σs basal production rates λσs are indicated in the figure. (C) The dynamic response of σs to a 10-min pulse of stress demonstrates that queueing provides for a very fast and dynamic response. Importantly, the response is highly specific temporally, as σs only remains at a high level during the time that excess aberrant proteins are around. We assume the basal rate λσs=500 min−1, while λm=1000 min−1 during the pulse of stress but λm=0 min−1 otherwise. (Inset) The mean response amplitude of σs to a periodic stress is strongest, especially at fast frequencies, when the system is on average near balance or slightly overloaded. The rate λm is taken to be a constant plus a sinusoid with amplitude 100 min−1 and given frequency. (D) The adaptive response leads to positive correlations between σs and mistranslated protein levels for a broad range of parameters, peaking near the balance point (respectively colored dashed lines). Parameters are the same as those used in (B).