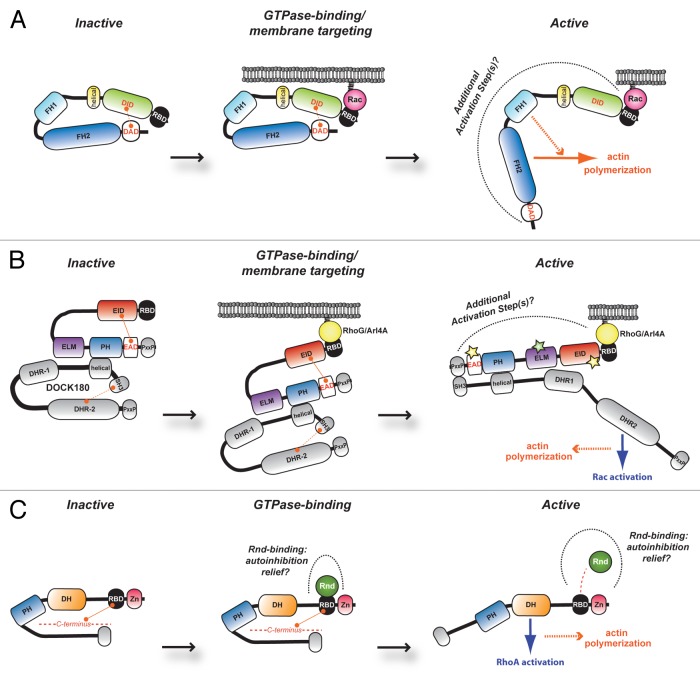
Figure 1. Representative models of FHOD1, ELMO family and Syx protein regulation. Schematic depicting proposed model of RBD-mediated regulation of (A) FHOD1, (B) ELMO family and (C) Syx. (A) At basal levels, the formin FHOD1 is repressed via intramolecular contacts between the DID and the DAD obscuring its actin nucleation function. RBD engagement by active Rac tethers the molecule at the cell membrane and relief of autoinhibition is suggested to occur through additional activation steps. A similar model of autoinhibition relief is proposed for (B) ELMO family proteins. These latter molecules are found constitutively in complex with DOCK proteins. It is suggested that binding of the ELMO RBD via active RhoG or Arl4A results in membrane targeting of the complex, release of DOCK180 autoinhibition (resulting in Rac activation through the DOCK GEF activity) and concomitant cytoskeletal reorganization. (C) In unstimulated conditions, it is hypothesized that Syx exists in an autoinhibited conformation through as yet undetermined portions in its N- and C-terminus. Relief of this closed conformation may come in the form of Rnd1/3-binding to the Syx RBD, thereby exposing its DH domain to catalyze nucleotide exchange specifically on RhoA and induce cytoskeleton rearrangement. RBD, Ras-binding domain; DID, diaphanous inhibitory domain; DD, dimerization domain; CC, coiled-coil region; FH, the actin nucleation module of formin-homology-1 and FH-2 regions; DAD, diaphanous autoregulatory domain; EID, ELMO inhibitory domain; ELM, ELMO homology; PH, pleckstrin homology; EAD, ELMO-autoregulatory domain; PxxP, proline-rich motif; DHR, DOCK homology region-1 and DHR-2; Zn, zinc-finger domain; DH, Dbl homology

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.