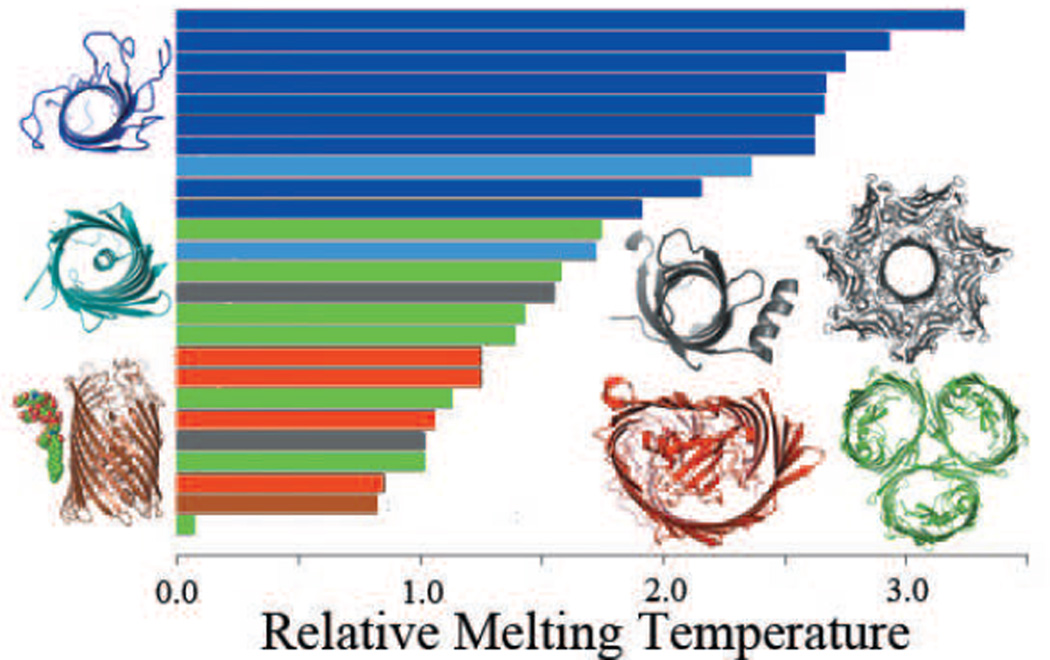
Figure 5.
The relative melting temperature of the transmembrane domains of 25 β-barrel membrane proteins can be calculated by enumerating all possible conformations in a reduced state space. Monomers that are stable without in-plugs and out-clamps, e.g., OmpA are shown in dark blue. Monomers stabilized by small in-plugs, e.g., NalP are shown in light blue. Monomers stabilized by out-clamps are represented by PagP and α-hemolysin (grey). β-barrels that require oligomerization for stability, e.g., ScrY are shown in green. Monomers stabilized by large in-plugs e.g FptA are shown in red. β-barrel membrane proteins can also have specific protein-lipid interactions, e.g., FhuA (brown) that increase protein stability. All stable monomers tend to have higher relative melting temperature and group towards the top of the graph (adapted from (127)).