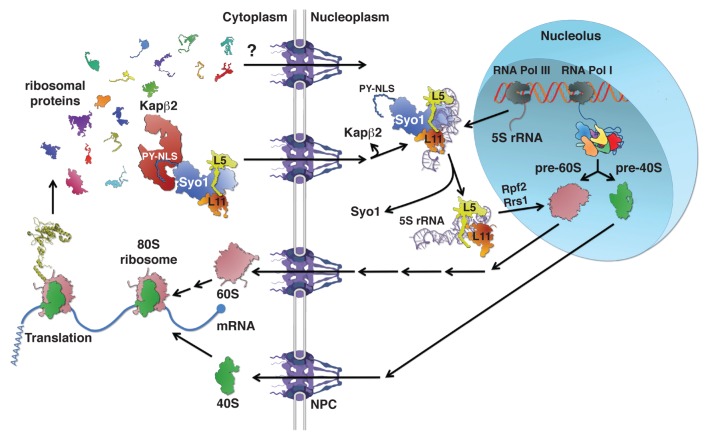
Figure 1. Symportin synchronizes nuclear import of the ribosomal proteins Rpl5 and Rpl11 with 5S RNP assembly. Ribosomal proteins (r-proteins) and ribosome biogenesis factors are synthesized in the cytoplasm and need to be imported into the nucleus before their assembly into pre-ribosomal particles. Passage across the nuclear pore complex (NPC) is facilitated by transport receptors of the importin-β family; however, in most cases the importin mediating nuclear import of a given r-protein or biogenesis factor is not yet known. Ribosome assembly starts in the nucleolus, a subnuclear compartment, by formation of a 90S pre-ribosomal particle upon transcription of the rDNA and the association of “early” r-proteins and biogenesis factors with the nascent 35S pre-rRNA. Concomitant to and/or after completion of transcription, endonucleolytic cleavage of the 35S pre-rRNA leads to the formation of pre-40S and pre-60S ribosomes. While pre-40S ribosomes are rapidly exported to the cytoplasm, the nuclear phase of pre-60S maturation is complex and involves several well-defined intermediates. The 5S RNP, composed of the 5S rRNA and the r-proteins Rpl5 and Rpl11, is incorporated into early pre-60S particles. The formation of the 5S RNP is already predetermined in the cytoplasm where Rpl5 and Rpl11 form an import complex with the transport adaptor Syo1. The trimeric Syo1-Rpl5-Rpl11 complex is recognized, via the N-terminal PY-NLS of Syo1, by the importin Kapβ2 (Kap104). Upon nuclear translocation and RanGTP-dependent cargo release, association of the 5S rRNA leads to the generation of a 5S RNP intermediate containing Syo1. Release of Syo1 is coupled to the formation of an assembly-competent 5S RNP that is most likely inserted with the aid of the biogenesis factors Rpf2 and Rrs1 into nucleolar pre-60S particles. Both pre-40S and pre-60S subunits undergo final maturation in the cytoplasm leading to acquisition of translation competence.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.