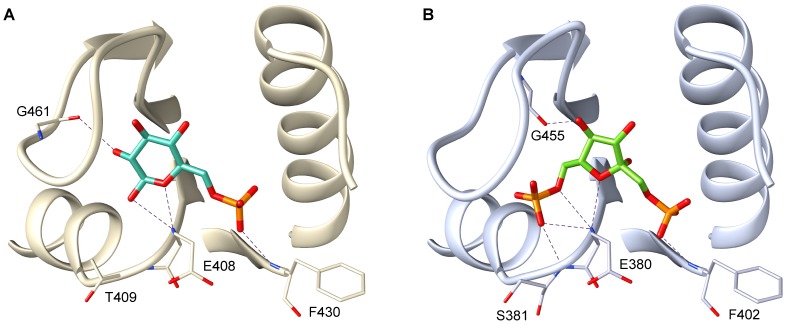
Figure 6. Docking poses of the ligands with the most favourable interaction energies for two modelled PYKs.
(A) G6P in the allosteric site of the Streptococcus pyogenes PYK. (B) FBP in the allosteric site of the Enterococcus faecalis PYK. The protein allosteric sites are shown in cartoon representation and the potential allosteric activators are shown in stick representation. The predicted allosteric activator FBP is bound in the allosteric site in its most favourable binding pose with a score of −81.4 kcal/mol. Hydrogen bonds between the ligands and the proteins (with a maximum length of 3.2 Å) are indicated by dashed lines.