Abstract
Purpose.
To characterize the features and possible mechanism of plus disease in the mouse oxygen-induced retinopathy (OIR) model for retinopathy of prematurity.
Methods.
Wild-type and Adam (A Disintegrin And Metalloproteinase) knockout mice were exposed to 75% oxygen from postnatal day 7 to 12 (P7 to P12) (hyperoxia), then returned to normal air (relative hypoxia). Live fundus imaging and fluorescein angiography at P17 were compared to immunofluorescence analysis of flat-mounted retinas. Two hallmarks of plus disease, arterial tortuosity and venous dilation, were analyzed on fixed retinas (P12–P17). The length of tortuous vessels was compared to a straight line between two points; the diameter of retinal vessels was determined using ImageJ software, and bromo-deoxyuridine (BrdU) labeling was used to visualize proliferation of retinal vascular cells.
Results.
Mice developed retinal arterial tortuosity and venous dilation after exposure to OIR, which was visible in live fundus images and fixed whole-mounted retinas. Vein dilation, arterial tortuosity, and BrdU incorporation gradually increased over time. Moreover, Adam8−/− and Adam9−/− mice and mice lacking Adam10 in endothelial cells were partially protected from plus disease compared to controls.
Conclusions.
The mouse OIR model can be used to study the pathogenesis of plus disease and identify potential therapeutic targets. The severity of plus disease increases over time following OIR and correlates with increased proliferation of endothelial cells, suggesting that proliferation of vascular cells may be a mechanism underlying the development of plus disease. Moreover, our findings suggest that ADAMs 8, 9, and 10 could be targets for treatment of plus disease.
Keywords: plus disease, oxygen-induced retinopathy, retinopathy of prematurity
Vascular tortuosity and dilation are critical diagnostic features of retinopathy of prematurity plus disease. In the mouse OIR model, the development of these vascular changes correlates with increased proliferation of vascular cells, suggesting that endothelial proliferation could contribute to plus disease.
Introduction
Retinopathy of prematurity (ROP) is a retinal vascular disease afflicting premature infants. Supplemental postnatal oxygen exposure has been identified as a significant etiological factor. After oxygen exposure, the ensuing relative hypoxia triggers a pathological neovascular response that results in formation of neovascular tufts and can lead to retinal detachment and loss of vision.1 Although ROP is treatable with laser photocoagulation if diagnosed at an early stage, it continues to be a leading cause of childhood blindness. In the United States, nearly 1500 infants develop treatment-requiring ROP each year, of whom approximately 400 to 600 suffer a lifetime of blindness (provided in the public domain by http://www.nei.nih.gov/health/rop/). In developing countries that are increasingly providing better neonatal care with improved survival rates of premature infants, the incidence of severe ROP is rising dramatically.2,3 Overall, the increased incidence of premature births and the insufficient supply of adequately trained ophthalmologists have exacerbated the challenges caused by the increasing number of ROP patients, who face the prospect of severe clinical and economic hardship as a consequence of their ROP.
Multicenter trials sponsored by the National Institutes of Health have shown that presence of plus disease is the critical diagnostic feature to identify infants with severe treatment-requiring ROP. Plus disease is defined as abnormality of the retinal vessels in which the degree of arterial tortuosity and venous dilation in the posterior pole exceeds that in a standard published photograph.4–7 The first detectable abnormalities are a slight dilation of the retinal veins, although the veins can be triple their normal size in the most severe cases. Vascular tortuosity is also considered a diagnostic landmark to define the severity of ROP, as the degree of tortuosity correlates with disease severity.8 Improved understanding of the pathogenesis of plus disease may permit earlier diagnosis, better treatment, and more precise monitoring of disease progression.
Currently, the venous dilation and arterial tortuosity that are hallmarks of plus disease are thought to be caused by reduced capillary resistance and increased blood flow to developing arteriovenous shunts.8 In this model, veins are thought to dilate because they offer lower resistance, whereas arterioles are thought to become tortuous because they are too rigid to dilate.8 Although this hypothesis has found substantial support in the literature,9 other studies have yielded no evidence for increased blood flow through the ophthalmic and central retinal arteries in eyes with plus disease.10,11 Moreover, plus disease occurs in cases of aggressive posterior ROP without obvious neovascularization or peripheral shunting.6,8,12 On the other hand, Hartnett et al. showed that anti-VEGF treatment decreased vessel tortuosity and dilation in a rat oxygen-induced retinopathy (OIR) model using 50%/10% oxygen cycling, suggesting that VEGF could mediate the changes in retinal vessels, perhaps by stimulating proliferation or migration of endothelial cells or both.12
Clinical plus disease diagnosis is defined based on appearance of the retina at a single time point only, and little is currently known about the initiation and progression of plus disease.13,14 Mouse models of OIR have been instrumental in identifying new targets for ROP treatment,1,15 and development of vascular tortuosity has been reported in this model16; yet much remains to be learned regarding the occurrence or progression of plus disease in mouse OIR. The main goal of this study was to characterize the vascular changes in mice exposed to OIR, to compare them to those observed in plus disease, and to explore the underlying mechanism. We evaluated the development of vascular tortuosity and dilation during mouse OIR and analyzed how intravitreal injection of bevacizumab (Avastin) or targeted deletions of members of the ADAM (A Disintegrin And Metalloproteinase) family of membrane-anchored metalloproteinases, previously implicated in pathological retinal neovascularization,17–20 affect the development of plus disease in the OIR model.
Materials and Methods
Reagents
All reagents were from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. FITC-isolectin B4 was from Vector Labs (Burlingame, CA), anti-bromo-deoxyuridine (BrdU)-biotin from Millipore (Billerica, MA), and Texas Red anti-biotin from Jackson Immunoresearch (West Grove, PA).
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All mice used in this study were of mixed genetic background (C57Bl/6J/129/Sv). For ADAM knockout mice, the corresponding wild-type littermates served as controls.
Fundus Photography and Fluorescein Angiography
To obtain fundus images from retinas from control and OIR-treated mice at postnatal day 17 (P17), the animals were anesthetized by intraperitoneal injection of ketamine (10 mg/kg) and xylazine (1 mg/kg), and the eyes were dilated with a drop of phenylephrine hydrochloride ophthalmic solution (Falcon Pharmaceuticals, Fort Worth, TX). The eyes were kept moist with carboxymethylcellulose sodium (Celluvisic; Allergan, Irvine, CA), and the retina was visualized using a Micron III camera (Phoenix Research Laboratories, Pleasanton, CA). For retinal angiography, mice were injected with 0.2% fluorescein 2 minutes before imaging.
Evaluation of Vascular Tortuosity and Dilation After OIR
Mice were exposed to the OIR model as previously described,17–20 and the progression of vascular changes was monitored at different time points. The tortuosity in the major vessels of the retina was quantified by tracing a line along the tortuous vessel and comparing it to a straight line traced from the vessel origin at the optic nerve to the first branch point (ImageJ software; NIH, Bethesda, MD). To assess vessel dilation, the diameter of the main central retinal vessels was measured at three points (at the point closest to and farthest from the optic nerve and in the middle between these two points) using ImageJ software, and the average diameter per vessel was compared in OIR-treated and untreated mice. Each sample was scored independently by three separate individuals in a masked manner. The unpaired Student's t-test (equal variation, two-sided) was used for statistical analysis, with P ≤ 0.05 considered statistically significant.
Evaluation of Cell Proliferation by BrdU and EdU Staining
Mice exposed to the OIR model were injected with 0.1 mg/g 5-bromo-2-deoxyuridine or 5-ethynyl-2-deoxyuridine (EdU) dissolved in sterile water21 and were humanely killed 2 hours after injection. For BrdU, the eyes were fixed in 4% paraformaldehyde (PFA) on ice for 3 minutes, then transferred to 70% ethanol and stored at −20°C for 2 hours. They were then washed in decreasing concentrations of ethanol for 10 minutes each, dissected in PBS, and washed for 30 minutes in 1 mL PBS/1% Triton X-100 on a horizontal shaker at low speed. The retinas were incubated at 37°C for 1 hour with 2 N HCl, washed in 1 mL 0.1 M sodium borate twice for 15 minutes, incubated overnight at 4°C with biotin-conjugated anti-BrdU antibody diluted 1:300 in PBS containing 1% BSA, and then washed three times with PBS–1% Triton X-100 for 5 minutes while being shaken at low speed. Retinas were incubated in Texas Red–labeled anti-biotin diluted 1:500 in PBS, 1% BSA for 2 hours with shaking while shielded from light, then washed twice in 1 mL PBS–1% Triton for 5 minutes each. Vessels were stained with FITC-isolectin B4 and mounted using antifade medium. Cellular proliferation was analyzed in all major central vessels of each retina by counting the number of BrdU-positive red-stained nuclei per length of vessel, expressed as number of positive nuclei per 250 μm vessel length. For EdU, eyes were fixed in PFA for 2 hours; the retinas were then isolated and stained using the Clik-iT kit following the manufacturer's instructions (Molecular Probes, Eugene, OR) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and FITC-isolectin B4.
Intravitreal Injection
OIR-treated mice and untreated controls received intravitreal injections of 2 μL 25 mg/mL bevacizumab (Avastin; Genentech, South San Francisco, CA) or 2 μL PBS as control into the right eye at P12; the left eye was untreated. The eyes were removed at P15 and P17 and immunostained as described above.
Results
Live Fluorescence Fundus Imaging After OIR Correlates With Fixed Retina Images
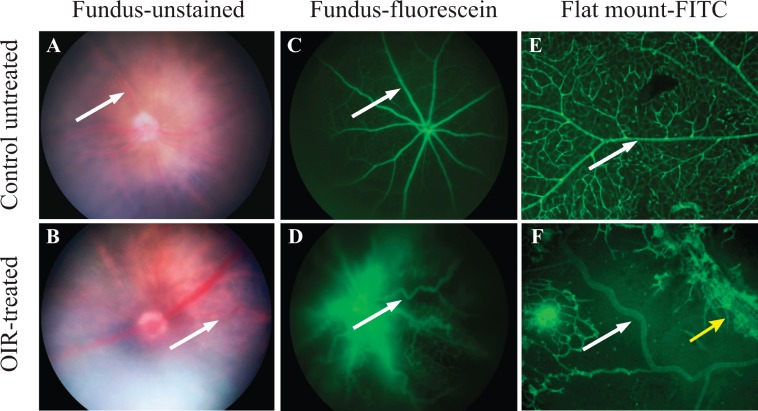
The major goal of this study was to determine whether the mouse OIR model might be suitable for studying the pathogenesis of “plus disease.” In order to evaluate tortuosity and dilation of retinal vessels at the conclusion of the mouse OIR model, P17 mice were anesthetized and their fundi were visualized with a Micron III camera (Phoenix Research Laboratories) (Figs. 1A, 1B; arrows point toward a straight vessel in an untreated control in Fig. 1A and toward a tortuous vessel in an OIR retina in Fig. 1B). Subsequently, these animals underwent fluorescein angiography to better visualize the retinal vasculature in vivo under blue light illumination (Figs. 1C, 1D). The normal retinal vasculature was clearly visible in the retina of an untreated mouse (Fig. 1C), and tortuous vessels were evident in the OIR-treated animal (Fig. 1D).
Figure 1.
The hallmarks of plus disease, vessel dilation and tortuosity, are replicated in the mouse OIR model. The normal distribution and shape of retinal vessels in untreated mice (A, C, E) are strongly altered following the OIR treatment (B, D, F). OIR-dependent vessel dilation and tortuosity become apparent when visualized through live fundus imaging and live fluorescein angiography (B, D). Immunofluorescent images of fixed retinal flat mounts stained with FITC-isolectin B4 to visualize endothelial cells demonstrate the dilation and tortuosity of the major central retinal vessels in the central avascular area of an OIR-treated retina ([F]: white arrow, artery; yellow arrow, vein) as compared to an untreated control (E). These images are representative of n = 10 for live fundus images and n = 10 for fixed flat-mounted samples.
Immunofluorescence Analysis of Vascular Tortuosity and Dilation on Fixed Retinas
Following the live imaging studies, the animals were humanely killed and their retinas processed for flat-mount staining with FITC-isolectin B4 to mark endothelial cells (Figs. 1E, 1F). The retina from an untreated control showed normal vessels with no evidence of tortuosity or dilation (Fig. 1E, arrow). However, the retina from an OIR-treated animal had enlarged and tortuous major vessels (Fig. 1F, arrows: artery, white; vein, yellow). The comparable retinal images obtained with live fundus imaging and with immunofluorescence on fixed retinas validate the use of fixed whole-mounted retinas to study the development of vascular tortuosity in mice, and rule out that the observed tortuosity is an artifact of fixation.
Time Course of the Development of Vascular Tortuosity
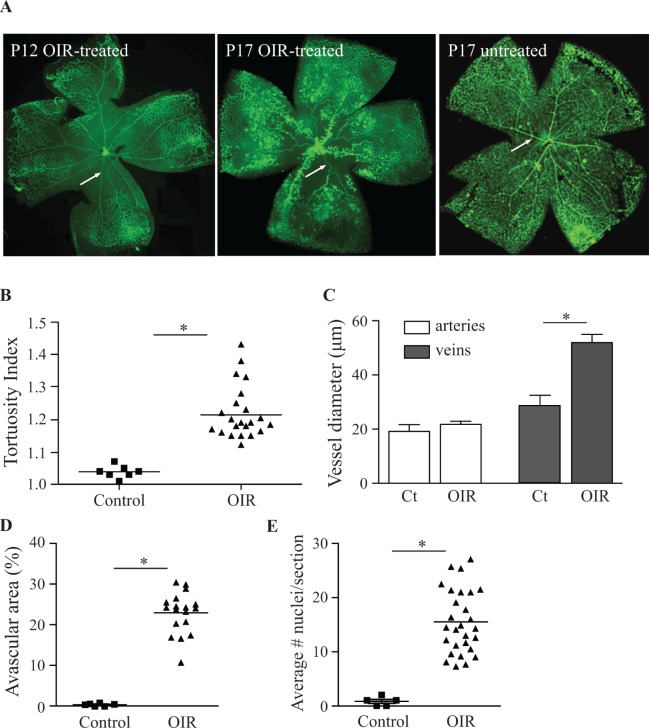
The presence of tortuous and dilated vessels in mouse retinas following OIR allowed us to assess different stages of the development of vascular tortuosity. After 5 days' exposure to 75% oxygen (P12), the major arteries and veins traversing the central avascular area were straight, without any detectable tortuosity, just as in untreated controls (Fig. 2A, arrow). At P17, the size of the central avascular area of OIR-treated mice was smaller due to partial revascularization of this area, and the major vessels in this area showed clear dilation or tortuosity (or both), which was not the case in untreated controls (Fig. 2A, arrow). Quantification demonstrated that mice exposed to the OIR model developed significant tortuosity of the major central retinal arteries at P17 (Fig. 2B). Moreover, the diameter of major veins was almost doubled, whereas arteries were not significantly dilated after OIR (Fig. 2C). The increased arterial tortuosity and dilation of veins coincided with an enlarged central avascular area (Fig. 2D) and an increased number of endothelial cell nuclei on the vitreal side of the internal limiting membrane (Fig. 2E), although there was no direct association between the degree of arterial tortuosity and the size of the central avascular area in individual eyes (data not shown).
Figure 2.
Significant arterial tortuosity and venous dilation are present in retinas of mice exposed to OIR. The retinas of mice exposed to the OIR model and untreated controls were flat mounted and stained with FITC-isolectin B4, a marker for endothelial cells. In OIR-treated mice at P12 (immediately after hyperoxia treatment), the major vessels traversing the central avascular area were straight and without evidence for dilation ([A] left). At P17, the size of the central avascular area was decreased, and vessel sprouts were visible along the dilated central veins. The presence of tufts and vascular tortuosity, as well as the dilation of veins, was clearly evident when compared to what was seen in untreated P17 control mice ([A], middle and right). The quantification of vascular tortuosity (see Materials and Methods for details) showed a significant increase at P17 in mice exposed to OIR compared to untreated controls (B). Measurements of vessel diameter demonstrated that arteries were not dilated in OIR-treated or control mice, whereas veins showed a significant increase in dilation at P17 (C). Ct, controls. The quantification of vaso-obliteration and neovascularization showed a significant increase in the size of the central avascular area (D), as well as in the numbers of endothelial cells that crossed the internal limiting membrane (E), following OIR treatment compared to no treatment. Data represent means ± SEM; *P ≤ 0.05. Images are representative of n = 7 for untreated controls, n = 22 for OIR-treated samples.
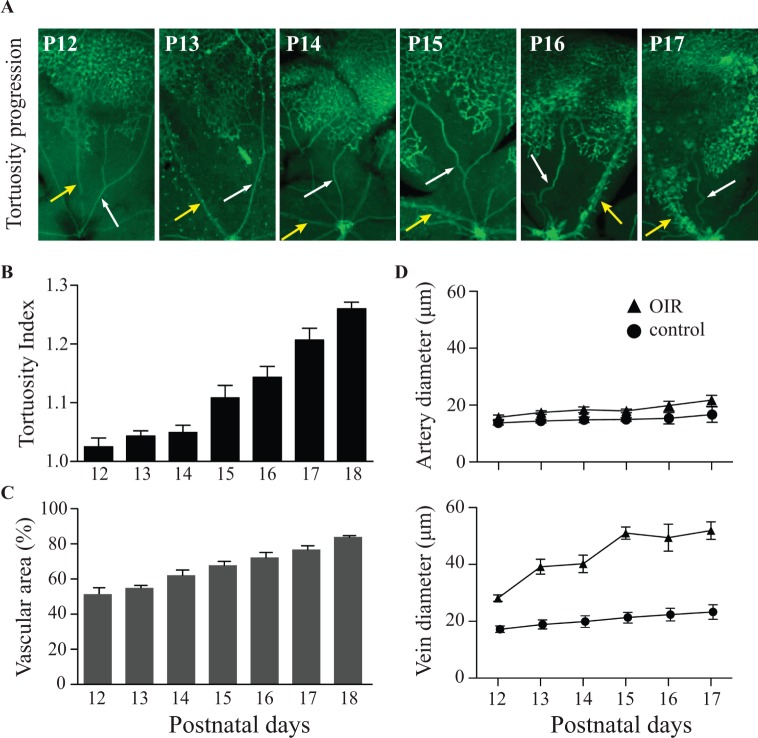
Next, we established the time course of the development of vascular tortuosity and the increase in vessel diameter following removal of the animals from the oxygen chamber at P12 (Fig. 3). The arteries and veins in the central avascular area were straight and had normal diameters at P13 and P14 (Fig. 3A, arrows: artery, white; vein, yellow). Vascular tortuosity and dilation of the large central vessels first became apparent at P15 and increased progressively until P17 (Fig. 3A). Quantification of the vascular tortuosity revealed a highly significant increase from P15 to P18 following OIR (Fig. 3B), whereas age-matched untreated controls did not develop tortuous vessels (not shown). The increased tortuosity coincided with the revascularization of the central avascular area (Fig. 3C). Quantification of the diameter of veins showed an increase starting at P13, an earlier stage compared to the increase in the tortuosity of arteries. The diameter of veins peaked at P15 and did not significantly increase further between P15 and P17. The large central veins of OIR-treated mice dilated to approximately twice the diameter of veins in untreated animals; but unlike the arteries, they did not develop tortuosity and had no other major visible changes in their morphology. On the other hand, arteries of OIR-treated animals developed tortuosity but were not significantly dilated (Fig. 3D).
Figure 3.
Progression of vascular tortuosity and dilation in mice exposed to the OIR model over time. The progression of vascular changes following the OIR model was analyzed in FITC-isolectin B4–stained flat-mounted retinas from P12 to P17. The dilation of veins first became apparent at P13, and was clearly visible at the end of the OIR treatment at P17 ([A]: white arrows, arteries; yellow arrows, veins). The quantification of the tortuosity index of arteries (see Materials and Methods for details) showed a time-dependent increase in the central vessels starting at P15 and peaking at P18 ([B]: controls, n = 3; OIR, n = 5). The quantification of the size of the central avascular area demonstrated a constant decrease in size from P12 to P18 (C), which correlated with the increase in tortuosity (B). The first evidence for dilation of veins was visible at P13, and vein dilation peaked at P15 and remained constant thereafter. Arteries did not show evidence for significant dilation at any time point compared to untreated controls ([D]; controls: arteries, n = 3, veins, n = 3; OIR: arteries, n = 5, veins, n = 5). All images are representative of five samples.
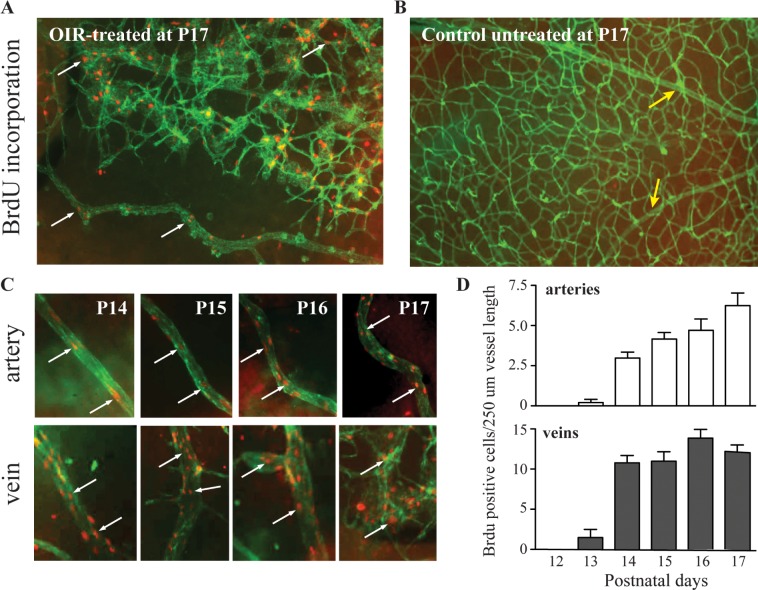
Increased BrdU Incorporation in Tortuous and Dilated Vessels
The increased tortuosity of retinal arteries and the enlarged diameter of retinal veins raised questions about the underlying cause. To assess whether these vascular changes could be brought about by increased proliferation of endothelial cells, we measured incorporation of the nucleotide analogue BrdU in endothelial cells as a proliferation marker following exposure to OIR. We found a significant increase in BrdU-labeled cells in tortuous retinal arteries and dilated veins after OIR, but no BrdU incorporation in retinal vessels of untreated controls (Figs. 4A, 4B). BrdU incorporation in small vessels and in the major central vessels of the retina in OIR-treated mice was clearly visible as early as P14. At this time point, there was no evidence for arterial tortuosity, whereas the veins had begun to dilate (see Fig. 3C). Quantification of BrdU incorporation showed that cell proliferation was first detectable at P13, increased substantially by P14, and continued to increase until P17 (Fig. 4D). The association of vascular tortuosity and dilation with increased proliferation of endothelial cells in the existing vessels raises the interesting possibility that endothelial cell proliferation may be a significant cause of the observed vascular tortuosity and dilation.
Figure 4.
Increased proliferation of endothelial cells is observed in tortuous vessels and dilated veins. Mice exposed to the OIR model or left untreated were injected with the nucleotide analogue BrdU to measure proliferation in retinal cells, with an emphasis on endothelial cells, as described in Materials and Methods. FITC-isolectin B4 was used to stain the retinal vessels (green), and cells with BrdU incorporation are shown in red. In mice exposed to the OIR, significant BrdU incorporation was present in small vessels as well as in the major dilated and tortuous central vessels at the end of OIR ([A]; arrows point to cells with BrdU incorporation). Untreated mice had a normal distribution and shape of the major central retinal vessels without BrdU staining ([B]; yellow arrows point to major central vessels). The time-course analysis of OIR-treated mice showed increased BrdU staining in arteries and veins (C). The straight arteries seen at P14 had few stained nuclei; but as OIR progressed, both the tortuosity and number of BrdU-positive cells increased ([C]; white arrows indicate BrdU staining). Dilation of veins also correlated with increasing BrdU staining of nuclei in endothelial cells, but only veins showed branching points that elongated as cell proliferation increased (C). The quantification of the number of cells stained with BrdU per 250-μm vessel length showed that proliferating cells were visible at P13 in both types of vessels but that veins showed increased proliferation compared to arteries at later time points, peaking at P14 and remaining similar until P17 (D). Arteries initially showed less proliferation, but this increased steadily over time until P17 ([D]; arteries, n = 10, veins, n = 10).
Intravitreal Injection of Bevacizumab Reduces Vascular Tortuosity and Dilation Following OIR
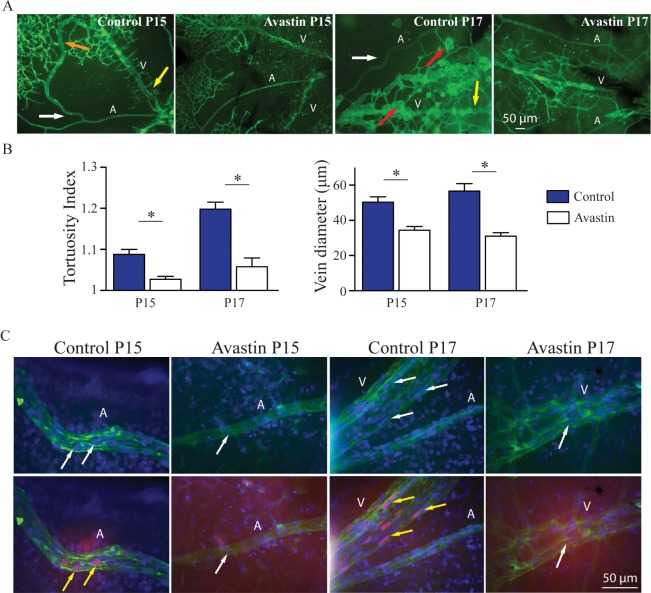
We next injected the VEGF-A antagonist bevacizumab intravitreally in mice subjected to OIR to assess the role of VEGF-A in the development of plus disease. We found a significant reduction of arterial tortuosity and vein dilation at P15 and P17 in bavacizumab-treated eyes compared to PBS-injected controls (Fig. 5A: artery, A; vein, V; orange arrow, vascular shunt in the control at P15; yellow arrow, neovascular tufts in the control at P17; white arrow, tortuous vessel, quantification in Fig. 5B). Finally, we showed that the nucleotide analogue EdU was incorporated into several endothelial cell nuclei in arteries and veins of control-injected eyes following OIR at P15 and P17, but not in vessels of bavacizumab-treated controls (Fig. 5C).
Figure 5.
Anti-VEGF treatment reduces arterial tortuosity and vein dilation. Mice exposed to the OIR model or left untreated were injected intravitreally with bevacizumab at P12, or with PBS as control. Arterial tortuosity and vein dilation were evaluated at P15 and P17. Representatives images of OIR-treated mice show arterial tortuosity (white arrow) and vein dilation (yellow arrow) at P15 and P17, and in some cases also formation of vascular shunts (orange arrow, [A], left) and vascular tufts (red arrows, third image from left). Mice receiving bevacizumab showed no evidence for vessel tortuosity or dilation or for tuft formation or vascular shunts at P15 or P17 (A). Quantification of arterial tortuosity and vein dilation demonstrated a significant reduction of both parameters in the bevacizumab-treated eyes compared to PBS-treated controls at P15 and P17 (B). Immunostaining of the vessels (see Materials and Methods) with FITC-isolectin B4 (green), DAPI (blue nuclei, indicated by white arrows), and EdU (magenta nuclei that colocalize with DAPI, indicated by yellow arrows) showed increased endothelial cell proliferation in the OIR-treated eyes injected with PBS (controls), whereas no arterial tortuosity, vein dilation, or endothelial proliferation was observed in the eyes injected with bevacizumab ([C]: control P15, n = 4; Avastin P15, n = 9; control P17, n = 4; Avastin P17, n = 9). Data represent averages ± SEM; P values were determined using Student's t-test, *P ≤ 0.05.
Development of Vascular Tortuosity in ADAM Knockout Mice
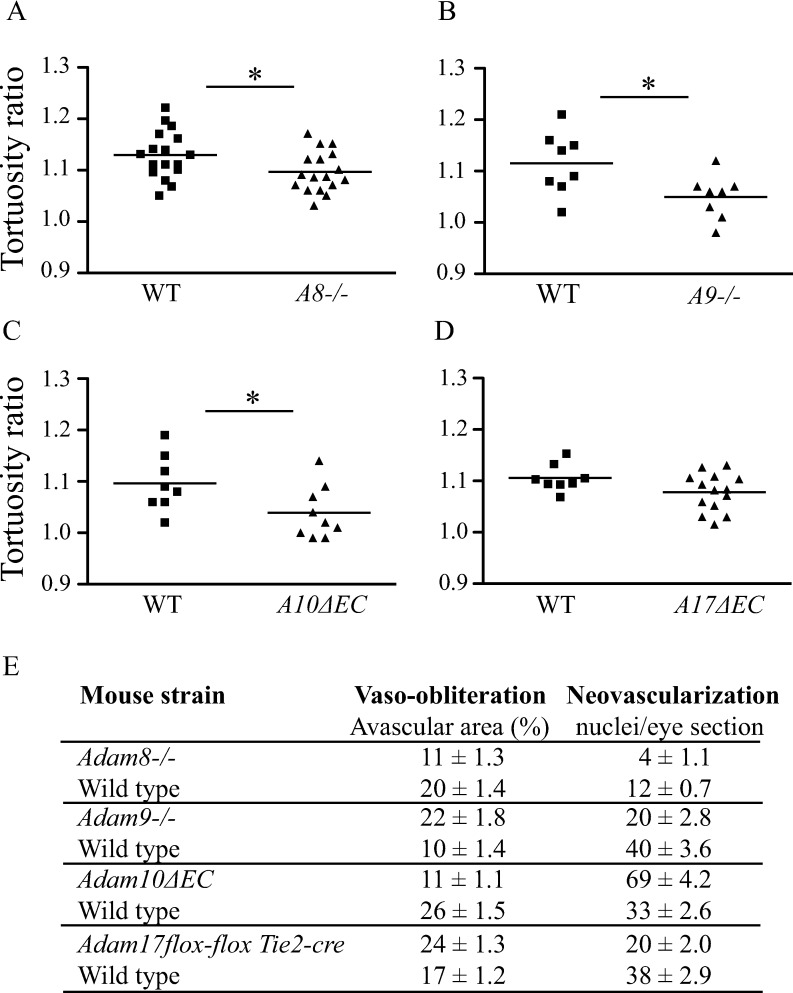
The highly reproducible time course of the development of arterial tortuosity and dilated veins in the mouse OIR model that resembled plus disease provided an opportunity to evaluate how inactivation of cell surface metalloproteinases of the ADAM family affect the development of vascular tortuosity as one key diagnostic criterion for plus disease. As shown in Figure 6, we found a significantly decreased vascular tortuosity in Adam8−/− and Adam9−/− mice following OIR, as well as in conditional knockout mice lacking ADAM10 in endothelial cells (A10ΔEC) (Figs. 6A–C). However, inactivation of ADAM17 in endothelial cells did not significantly affect the development of vascular tortuosity compared to what was seen in littermate controls, although there was a trend toward a lower response (Fig. 6D). The effects of the targeted deletion of these ADAMs on size of the central avascular area and the formation of neovascular tufts are shown in Figure 6E.
Figure 6.
OIR-induced plus disease is dependent on ADAMs. The contribution of ADAMs to the development of vascular tortuosity following OIR was evaluated in Adam8−/− and Adam9−/− knockout mice, as well as in conditional knockout mice lacking ADAM10 or ADAM17 in endothelial cells (Adam10ΔEC or Adam17ΔEC mice), with wild-type littermates serving as controls. All animals were exposed to the OIR model and the retinas evaluated for vascular tortuosity at P17, as described in Materials and Methods. Adam8−/− ([A]: n = 16; controls, n = 16) and Adam9−/− ([B]: n = 8; controls, n = 8) mice, as well as Adam10ΔEC mice ([C]: n = 8; controls, n = 9), had significantly less vascular tortuosity than their wild-type controls. The Adam17ΔEC mice ([D]: n = 8; controls, n = 14) mice, however, did not display significant changes in their vascular tortuosity level after OIR. The vaso-obliteration and tuft formation were evaluated in the ADAM knockout mice as well their corresponding littermate controls ([E]; these results are taken from the original publications on the analysis of pathological retinal neovascularization in these animals, see Refs. 17–20 for details). Data represent average ± SEM; P values were determined using Student's t-test, *P ≤ 0.05.
Discussion
Retinopathy of prematurity is a progressive neovascular disease affecting the eyes of premature infants. In the most severe form of ROP, these vascular changes include abnormal dilation and tortuosity of the retinal blood vessels, which are considered hallmarks of “plus disease.” The diagnosis of plus disease is based on a single evaluation, and there can be significant disagreement on the validity of this diagnosis, even among experienced ophthalmologists.22 Currently, the main hypothesis regarding the cause of plus disease is that it originates as the result of reduced capillary resistance and increased retinal blood flow to developing arteriovenous shunts.8 In kittens exposed to ROP, pericytes reportedly adopt a phenotype associated with increased vascular instability, suggesting a decreased ability to regulate blood flow, which in turn was proposed to cause the enlargement and tortuosity of the retinal vessels.23 However, hemodynamic parameters of retinal blood flow in children with plus disease showed no correlation with the progression of the disease.10,11 An alternative explanation for the mechanism underlying the development of plus disease is that the increased production of VEGF-A may act directly on central retinal blood vessels and cause dilation and tortuosity. This could explain why plus disease occurs before obvious peripheral shunting and neovascularization in some cases of aggressive posterior ROP.8
Here, we used the mouse OIR model to assess whether this model could provide new information about the pathogenesis of ROP. Moreover, we tested whether it could help to identify new targets for treatment of plus disease by allowing studies in genetically modified mice. We found that venous dilation and arterial tortuosity develop over time, consistent with the clinical observation that plus disease is progressive. Interestingly, the development of tortuosity in arteries and the dilation of veins in the OIR mouse model correlated strongly with increased endothelial cell proliferation in both types of vessels. The proliferation of endothelial cells in veins presumably leads to their dilation because veins lack pericyte ensheathment and can therefore expand in width. On the other hand, arteries are surrounded by pericytes and an extracellular matrix, and are therefore presumably unable to accommodate rapid growth in diameter. Instead, they may become tortuous as a result of the increasing number of endothelial cells.
Based on these findings, we hypothesized that the dilation of retinal veins and tortuosity of arteries following OIR are a consequence of higher levels of proliferation of endothelial cells, most likely because of the increase in VEGF that is triggered by relative hypoxia during OIR.24–28 This hypothesis is directly supported by our observation that intravitreal injection of the VEGF-A antagonist bevacizumab strongly reduced the development of vascular tortuosity in the OIR model, consistent with a recent report by Rabinowitz et al.,29 and prevented dilation of veins as well as proliferation of endothelial cells. This model also suggests that plus disease should correlate with the level of VEGF produced during the hypoxic phase of ROP. Interestingly, several studies have found that VEGF levels fluctuate during the different stages of ROP. The stronger the hyperoxic trigger is during phase I ROP (hyperoxic stage), the higher is the VEGF production in phase II ROP (hypoxic stage), resulting in more aggressive plus disease.1,30 Additional evidence for the role of VEGF in ROP plus disease comes from the interactions of other growth factors that are maternally produced and that interact with VEGF. Premature birth leads to early suppression of VEGF and decreasing levels of IGF-1; but during the proliferative phase of ROP, VEGF levels increase along with rising levels of IGF-1, thereby presumably promoting abnormal neovascularization.30 With these findings taken together, anti-VEGF-A treatment for ROP should therefore most likely also be effective for promoting regression of plus disease in patients.
Plus disease is a clinically important part of ROP; therefore an assessment of plus disease in the mouse OIR model could be relevant for identifying new targets for treatment of ROP, especially as it is an early change that could be treated before more visually threatening and permanent sequelae occur. The development of vascular tortuosity and dilation resembling ROP plus disease in the mouse OIR model provided the opportunity to test the development of plus disease in genetically modified mice. Previous studies from our lab have uncovered distinct roles for several ADAMs (ADAMs 8, 9, 10, and 17) in pathological retinal neovascularization using the mouse OIR model.17–20 Here, we tested how inactivation of these ADAMs affects the outcome of plus disease, with an emphasis on arterial tortuosity. At the conclusion of the OIR model at P17, Adam8−/− and Adam9−/− mice as well as mice lacking ADAM10 in endothelial cells displayed significantly less tortuosity than their wild-type littermates; in contrast, mice lacking ADAM17 in endothelial cells showed only a trend toward reduced tortuosity, but the change was not statistically significant. These studies suggest that ADAMs 8, 9, and 10 could be considered potential targets for treatment of plus disease in ROP. Taken together, our results highlight the potential usefulness of the mouse OIR model to explore the mechanisms underlying plus disease and to identify new targets for its treatment.
Acknowledgments
Supported by the Hickey Foundation (CPB, VHG), National Institutes of Health Grant EY19474 (MFC), and unrestricted departmental funding from Research to Prevent Blindness (NJH, MFC, MIR, RVPC). NJH was also supported by a research grant from the Dr. Werner Jackstaedt Foundation, Wuppertal, Germany.
Disclosure: V.H. Guaiquil, None; N.J. Hewing, None; M.F. Chiang, None; M.I. Rosenblatt, None; R.V.P. Chan, None; C.P. Blobel, None
References
- 1. Heidary G, Vanderveen D, Smith LE. Retinopathy of prematurity: current concepts in molecular pathogenesis. Semin Ophthalmol. 2009; 24: 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sylvester CL. Retinopathy of prematurity. Semin Ophthalmol. 2008; 23: 318–323 [DOI] [PubMed] [Google Scholar]
- 3. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008; 84: 77–82 [DOI] [PubMed] [Google Scholar]
- 4. Early Treatment For Retinopathy Of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121: 1684–1694 [DOI] [PubMed] [Google Scholar]
- 5. Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007; 125: 875–880 [DOI] [PubMed] [Google Scholar]
- 6. International Committee for Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123: 991–999 [DOI] [PubMed] [Google Scholar]
- 7. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics. 1988; 81: 697–706 [PubMed] [Google Scholar]
- 8. Davitt BV, Wallace DK. Plus disease. Surv Ophthalmol. 2009; 54: 663–670 [DOI] [PubMed] [Google Scholar]
- 9. Solarte CE, Awad AH, Wilson CM, Ells A. Plus disease: why is it important in retinopathy of prematurity? Middle East Afr J Ophthalmol. 2010; 17: 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niwald A, Gralek M. Evaluation of blood flow in the ophthalmic artery and central retinal artery in children with retinopathy of prematurity. Klin Oczna. 2006; 108: 32–35 [PubMed] [Google Scholar]
- 11. Neely D, Harris A, Hynes E, et al. Longitudinal assessment of plus disease in retinopathy of prematurity using color Doppler imaging. J AAPOS. 2009; 13: 509–511 [DOI] [PubMed] [Google Scholar]
- 12. Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008; 49: 3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelman SK, Gelman R, Callahan AB, et al. Plus disease in retinopathy of prematurity: quantitative analysis of standard published photograph. Arch Ophthalmol. 2010; 128: 1217–1220 [DOI] [PubMed] [Google Scholar]
- 14. Thyparampil PJ, Park Y, Martinez-Perez ME, et al. Plus disease in retinopathy of prematurity: quantitative analysis of vascular change. Am J Ophthalmol. 2010; 150: 468–475 e462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008; 84: 83–88 [DOI] [PubMed] [Google Scholar]
- 16. Furtado J, Davies M, Choi D, et al. Imaging retinal vascular changes in the mouse model of oxygen-induced retinopathy. Transl Vis Sci Technol. 2012; 1: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glomski K, Monette S, Manova K, De Strooper B, Saftig P, Blobel CP. Deletion of Adam10 in endothelial cells leads to defects in organ-specific vascular structures. Blood. 2011; 118: 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guaiquil V, Swendeman S, Yoshida T, Chavala S, Campochiaro P, Blobel CP. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009; 29: 2694–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guaiquil VH, Swendeman S, Zhou W, et al. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J Mol Med. 2010; 88: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weskamp G, Mendelson K, Swendeman S, et al. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010; 106: 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol. 2008; 315: 72–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slidsborg C, Forman JL, Fielder AR, et al. Experts do not agree when to treat retinopathy of prematurity based on plus disease. Br J Ophthalmol. 2012; 96: 549–553 [DOI] [PubMed] [Google Scholar]
- 23. Hughes S, Gardiner T, Baxter L, Chan-Ling T. Changes in pericytes and smooth muscle cells in the kitten model of retinopathy of prematurity: implications for plus disease. Invest Ophthalmol Vis Sci. 2007; 48: 1368–1379 [DOI] [PubMed] [Google Scholar]
- 24. Xiang N, Zhao MJ, Li XY, Zheng HH, Li GG, Li B. Redundant mechanisms for vascular growth factors in retinopathy of prematurity in vitro. Ophthalmic Res. 2011; 45: 92–101 [DOI] [PubMed] [Google Scholar]
- 25. Zhao M, Shi X, Liang J, et al. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp Eye Res. 2011; 93: 921–926 [DOI] [PubMed] [Google Scholar]
- 26. McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004; 10: 512–520 [PMC free article] [PubMed] [Google Scholar]
- 27. Hu J, Song X, He YQ, et al. Heparanase and vascular endothelial growth factor expression is increased in hypoxia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2012; 53: 6810–6817 [DOI] [PubMed] [Google Scholar]
- 28. Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995; 92: 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabinowitz R, Priel A, Rosner M, Pri-Chen S, Spierer A. Avastin treatment reduces retinal neovascularization in a mouse model of retinopathy of prematurity. Curr Eye Res. 2012; 37: 624–629 [DOI] [PubMed] [Google Scholar]
- 30. Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol. 2009; 24: 82–86 [DOI] [PMC free article] [PubMed] [Google Scholar]