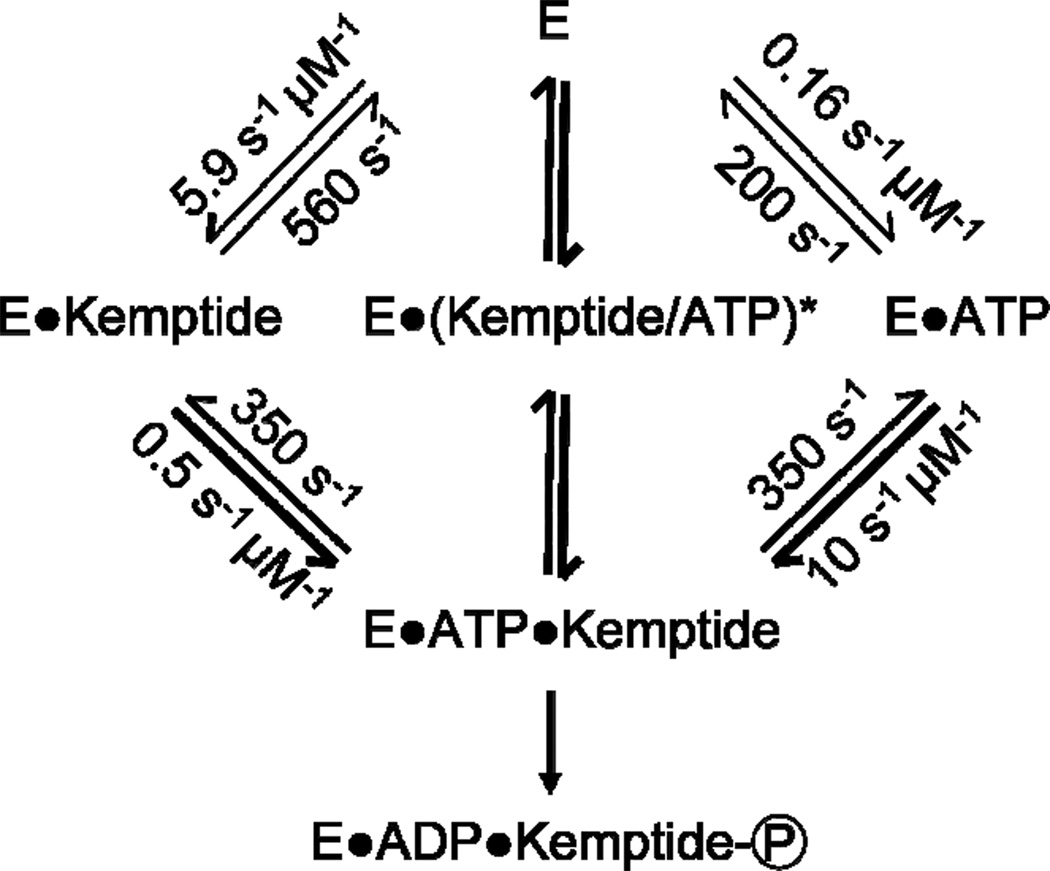
Figure 6.
A kinetic scheme for the PKA-catalyzed phosphoryla-tion of Kemptide with rates measured by a single PKA functionalized SWNT device. Second order rate constants for complex formation were calculated by dividing the observed rates by the appropriate substrate concentrations. The thickness of the lines reflects the transition probabilities from Table 2 with thicker lines indicating a higher probability. As described in the text, the intermediate marked with an asterisk is unobserved; formation and dissociation of the ternary complex by this route occurred with rates of 230 and 410 s−1, respectively.