Abstract
Background: Emergence of resistance was recognized shortly after the introduction of lamivudine. This 10 year retrospective study investigates resistance to lamivudine and the modifications of antiviral strategies required.
Patients and methods: Two hundred and nine patients were treated with lamivudine. Sixty seven out of 209 patients were excluded from the present study. HBVDNA was tested using the PCR assay and genotypic resistance was performed using the direct PCR sequencing.
Results: In the 125 patients initially treated with lamivudine monotherapy: Α) 48 (38.4%) patients with a mean time of 63.6±26.2 months under lamivudine treatment have normal ALT levels with negative (19%) or low (<1X102) HBVDNA levels, 10% developed cirrhosis, 1 HCC and 6% cleared HBsAg. Β) Resistance was developed in 61.60% patients within 45±23.84 months of lamivudine treatment. These patients were: 1) either switched to adefovir (9), entecavir (2) or tenofovir (2) or adefovir was added to lamivudine (21) for a short time and then they were switched to adefovir alone. Six out of 34 patients developed cirrhosis and 4 HCC while on treatment. 2) or adefovir was added-on to lamivudine (43). In 39 out of 43 treatment is ongoing while on virological response. No one developed cirrhosis or HCC. C) Seventeen patients received de novo combination therapy with lamivudine and adefovir and 2 out of 17 (11.7%) showed resistance to adefovir after 24 months of therapy.
Conclusions: Our results showed that a) approximately 38.4% of patients maintain viral suppression more than 5 years of lamivudine treatment and b) rescue therapy with add-on adefovir to ongoing lamivudine, seems to be a better treatment strategy associated with long term benefit regarding disease complications.
Keywords: Hepatitis B, treatment, lamivudine, adefovir, resistance, rescue therapy
Introduction
Chronic hepatitis B infection continues to be an important cause of morbidity, mortality and source of potential new infections worldwide1,2. The World Health Organization estimates that 400 million individuals are chronically infected with HBV. Furthermore, HBV is the 10th leading cause of death worldwide.
HBeAg-negative chronic hepatitis is common not only in Southern Europe, predominating in the Mediterranean area, but worldwide and is related to higher risk of disease progression, liver failure and high incidence of hepatocellular carcinoma. Progression to these complications is more frequent among patients who harbor HBV with mutations in its precore or core promoter regions3,4, although the majority of these patients may remain as inactive carriers for extended periods of time or during their entire lifetime. In a recent study conducted in Greece, HBeAg(-) hepatitis B was found to be the predominant form (92.1%) among Greek patients5.
The goals of therapy in patients with chronic HBV infection aim in limiting or reversing the progression of the disease through sustained viral suppression. Lamivudine was the first nucleoside analogue to be approved for chronic hepatitis B therapy. Long-term therapy with lamivudine was found to be initially effective, with high initial on treatment remission rates. However, the emergence of resistance was recognized shortly after the introduction of lamivudine in clinical practice, ranging from 23% in patients after 1 year, 46% after 2 years and 71% after 5 years, usually followed by biochemical breakthrough phenomena6.
In this 10 year (1999-2009), single center, open-label study, we retrospectively evaluated the safety, efficacy and resistance to lamivudine in patients with HBeAg-negative chronic hepatitis B, the changes in therapeutic strategies thereafter and the incidence of disease progression.
Patients and methods
This retrospective study included 209 patients with chronic HBeAg(-) hepatitis B (CHB) initially treated with lamivudine and followed-up between the years 1999 and 2009 in our center, 2nd Department of Medicine, in Aristotle University Medical School of Thessaloniki Greece. A centralized diagnostic index and laboratory database were used to identify all potential patients with these specific characteristics. Once the diagnosis of CHB and the exact study population was established, a retrospective chart review was performed in order to retrieve information regarding the long-term outcomes in these patients.
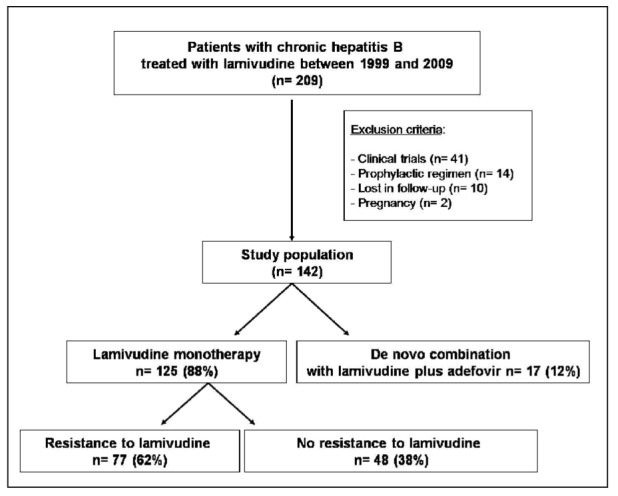
Sixty seven out of 209 patients were excluded from further analysis following medical history review for several reasons: Ten were lost to follow-up, 41 were already participants in clinical trials, 2 were treated during pregnancy and in 14 lamivudine was given as supportive therapy during immunosuppression. Seventeen out of 142 patients received de novo combination therapy of lamivudine with adefovir and 125 patients received lamivudine monotherapy. Eighty two patients out of 142 were IFN-α experienced in the past, having a primary non-response to IFN-a. They were subsequently treated with either lamivudine or lamivudine and adefovir. Figure 1 outlines the selection of patients in our study.
Resistance to lamivudine in this study was defined as the development of biochemical [ALT>1.5x upper limit of normal (ULN)] and virological (increasing HBV DNA≥ 1.0 log IU/mL) breakthrough in patients receiving lamivudine.
HBV DNA and liver biochemistries
HBV DNA values and liver biochemistries were obtained during routine visits while on standard treatment care of CHB. HBVDNA was measured using the Amplicor HBV test (Roche Diagnostics) (1999-2003) and Tacqman HBV (Roche Diagnostics) (2004-2009). The bi-directional PCR sequencing was used for genotype resistance (Trugene HBV, Siemens Diagnostics).
Baseline liver biopsy was performed in 67 patients prior to treatment initiation.
Major events
Cirrhosis was diagnosed based on clinical, histological, laboratory and radiologic evidence. Liver decompensation was diagnosed in case of development of ascites or bleeding or enchepathopathy. Hepatocellular carcinoma (HCC) was diagnosed based on high serum AFP levels, atypical findings on triple-phase computed tomography (CT) or magnetic resonance imaging (MRI). Cases with atypical imaging findings were confirmed with CT-guided biopsy.
Statistical Analysis
Baseline characteristics were calculated as the median (range) for continuous variables. The number and percent in each group were tabulated for categorical variables. The chi-square test of independence was used to determine statistical significance for categorical data. For the continuous variables, the Wilcoxon rank sum test was used. Primary endpoint of the study was the time to development of resistance to lamivudine.
Results
The patients' baseline characteristics are presented in Table 1.
Table 1. Baseline characteristics of study patients.
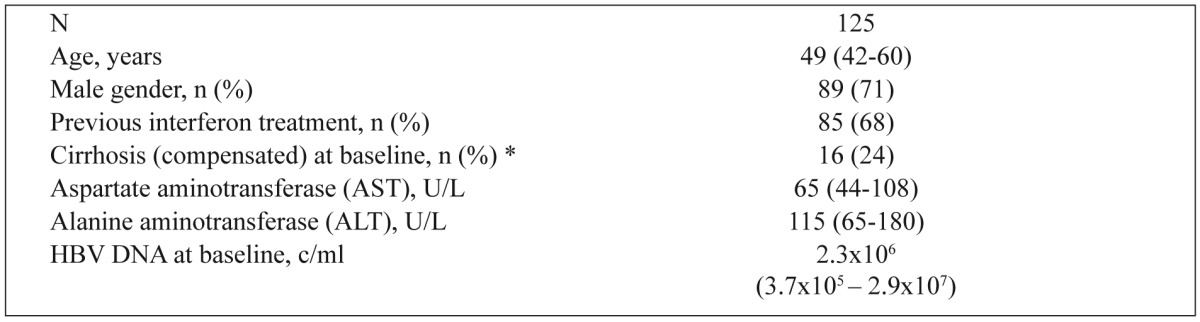
Data are presented as median (interquantile range) unless otherwise indicated. Normal ranges: AST(10-37 IU/L), ALT (10-45 IU/L). * Baseline liver biopsy was available in 43 and 24 patients, respectively.
Emergence of resistance to lamivudine
Resistance to lamivudine monotherapy was detected in 77/125 (62%) patients after a median time of 3.2 years (range= 0.5-9) following treatment initiation. Figures 1,3 show the occurrence of resistance over time. Resistance was confirmed with genotypic analysis in 50/77 patients. Simultaneous mutations at positions rtM204V/l and rtL180M were detected in 22 patients, mutation at position rtM204V/l in 25 patients and mutation at position rtL180M in 3 patients. Genotypic analysis was not possible for 27 patients in whom, however, compliance to treatment was confirmed.
Figure 1. Schematic patient chart enrolled in the study.
The remaining 48/125 (38%) patients on lamivudine monotherapy were followed for a median time of 5.4 years (range= 2-11) without any evidence of resistance.
No demographic or laboratory characteristic at baseline predicted the occurrence of resistance to lamivudine. However, patients that developed resistance to lamivudine had significantly greater mean HBV DNA value after 6 months of treatment as compared to those with no evidence of resistance (6.4x106 vs. 165, p< 0.0001).
Management of resistance
Table 2 summarizes the therapeutic strategies followed in patients with resistance to lamivudine monotherapy. Adefovir was added to lamivudine in 43 patients (56%), whereas in 34 (44%) patients the treatment regimen was switched to adefovir, entecavir or tenofovir. In 4/30 patients switched to adefovir with partial response to adefovir, entecavir was added or they were switched to entecavir or tenofovir. Resistance to adefovir was detected in 3/30 (10%) patients, following genotypic analysis. Simultaneous mutations at positions rtA181T/V and rtN236T were detected. The majority of patients, 39/43 (91%), with the addition of adefovir reached and sustained a complete virological response.
In addition, 1/17 (6%) patients who received de novo combination treatment of lamivudine with adefovir, showed resistance to adefovir after 24 months of therapy.
Long-term outcomes
Eighteen out of 125 patients presented to our center had cirrhosis at baseline. When these patients were analyzed according to treatment strategies and disease progression to major complications, it was shown that: a) During a 6-year follow-up, 1/48 patient without resistance to lamivudine, but with decompensated cirrhosis developed HCC, while 2/48 with cirrhosis, showed no further complications. b) Three out of 6 patients in the switch group, on adefovir or entecavir, with baseline decompensated cirrhosis, developed HCC. One patient with cirrhosis showed no disease progression and two other patients with liver decompensation died, one during liver transplantation procedure and the other due to non-hepatic reason. c) Five out of 5 patients in the add-on group showed no further complications, and d) In the group of 17 patients treated with de novo combination of lamivudine with adefovir, two of the four patients that presented with decompensated cirrhosis developed HCC.
Three out of 48 (6%) patients without resistance to lamivudine lost HBs antigen and sercoverted to antiHBs during follow-up. However, 5/48 (10%) patients developed cirrhosis and 1/48 (2%) developed HCC.
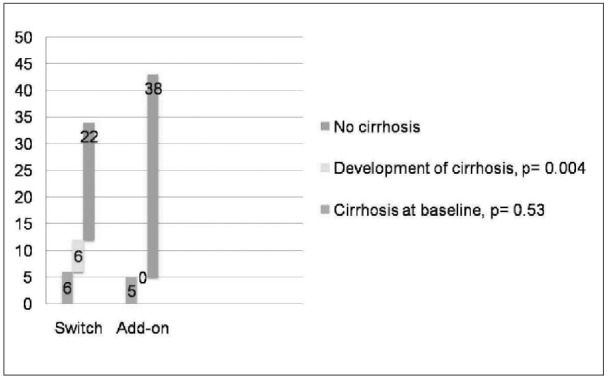
Patients who were switched to another drug following development of resistance to lamivudine, were more likely to develop cirrhosis when compared to those who were treated with adefovir addition (6 vs. 0, p= 0.004), while no significant difference in the prevalence of cirrhosis at baseline was noticed between these two groups (Figure 4). In addition, 4/34 (12%) patients who were switched to another drug developed HCC while on treatment.
Figure 4. Development of cirrhosis per therapeutic strategy for resistance to lamivudine.
Discussion
This retrospective study conducted in one academic center in Greece, evaluated 125 patients with HBeAg-negative chronic hepatitis B, initially treated with lamivudine and followed-up from 1999 to 2009.The objective of the study was to estimate the response to treatment, the emergence of lamivudine-resistant strains and their consequences, as well as the effect of two different treatment strategies thereafter: switch to adefovir or add-on adefovir.
When treating naïve patients, as far as disease progression is concerned, it was found that in a significant number of patients (18/125, 14.4%), CHB had already progressed to cirrhosis and decompensated cirrhosis (6/18, 33.3%), at the time they first presented to our center. This finding could be possibly explained by the fact that since CHB is asymptomatic , their disease could have been discovered incidentally during HBsAg screening of blood donors, asymptomatic ALT elevations or disease complications. Similar results have been shown by others, where 54% of patients with chronic hepatitis B during their first presentation, in 30% of them appeared with cirrhosis (CTP A) and 16% CTP B or C7, as well as in a previous study conducted in Greece5.
Our study showed that in 62% (77/125) of our patients initially treated with lamivudine monotherapy, resistance occurred after a median time of 3.2 years.
Lamivudine was the first oral drug to be approved in 1998 for the management of chronic HBV infection, followed by adefovir in 2002, entecavir in 2005, telbivudine in 2005 and finally with tenofovir in 2008. Lamivudine is a potent inhibitor of viral polymerase activity with an excellent safety profile. However, lamivudine monotherapy is not currently reccomended as a first-line treatment due to induction of high drug resistance, especially under long-term administration, with resistance developing in 23% of patients within 1 year, 46% after 2 years and 71% after 4 years6-10.
Nevertheless, it is also noteworthy that approximately 20% of patients maintain viral suppression during 5 years of lamivudine treatment11. In fact, our results showed that 38% of our patients with lamivudine monotherapy, followed-up for a median of 5.4 years, showed no evidence of resistance emergence. Similar results were also reported in other studies, with a rate of sustained virological response up to 39% after 4 years of lamivudine therapy. Patients with baseline cirrhosis who maintained virological response, were less likely than those with viral breakthrough to develop HCC and disease worsening7. The efficacy of lamivudine was also similar to what has been reported in another study from Greece, with approximately one third of the patients remaining in remission at 4 years of therapy12.
In our study, when patients with baseline cirrhosis were analyzed according to treatment strategies and disease progression, it was found that a) only one patient presented with decompensated cirrhosis who continued long-term lamivudine therapy, developed HCC and in 2 patients there was no disease progression b) 3/6 patients in the switch group developed HCC, 1 showed disease progression and the other 2 patients with baseline liver decompensation died, one during liver transplantation and the other due to non-hepatic reason and c) 5 patients with adefovir added to lamivudine, showed no further disease complications.
Furthermore, although several factors may possibly affect emergence of resistance, it is difficult to predict which patients will maintain suppression during lamivudine therapy. High baseline liver score (HAI), high body weight and high HBVDNA levels, as well as detectable HBVDNA at 6 months of lamivudine treatment are reported to be predictive of resistance emergence10. In our study, the patients' baseline demographic, laboratory or virological characteristics were not predictive the occurrence of resistance to lamivudine. However, patients who developed resistance to lamivudine, had a significantly higher viral load at 6 months after treatment initiation.
Adefovir dipivoxil, although having a moderate antiviral potency compared to other antivirals, was initially approved as a first-line therapy, but also as a rescue therapy for patients with lamivudine resistance. Adefovir treatment duration with a maximum of 240 weeks was reported to produce significant improvement in hepatic fibrosis, durable suppression of HBV and normal liver enzymes. However cumulative probability of HBV mutations after 240 weeks was 29%, more frequently seen among patients with high levels of HBVDNA at week 4813.
In the past, since adefovir was used as a salvage therapy to patients with resistance to lamivudine, there was a debate as to whether switch to adefovir or add-on adefovir to lamivudine was the best strategy. Subsequently, the results of long-term studies have proved and favored the add-on strategy as the best alternative, since no adefovir resistance was observed when adefovir was added on to ongoing lamivudine9. Fundamentally, very rarely adefovir resistance emerges within the first 3 years. In contrast, using adefovir as a switch therapy, led 25% of lamivudine-resistant patients to develop genotypic resistance to adefovir within 2 or 3 years of treatment14,15.
Following resistance to lamivudine, two different therapeutic strategies were implemented in our patients: switch to adefovir or add-on adefovir to lamivudine. Our results showed that add-on adefovir to lamivudine was a better treatment strategy compared to switch to adefovir or another antiviral based on the following: 4/30 patients switched to adefovir had a partial response and 3 of these (10%), developed resistance to adefovir, whereas the majority of patients with adefovir addition to lamivudine reached and sustained virological response and 5/5 patients with cirrhosis at baseline showed no further disease complications.
In the group of 17 patients who received de novo combination treatment of lamivudine with adefovir, only 1/17 patients showed resistance to adefovir after 24 months of therapy. However this treatment strategy is no longer recommended in naïve patients.
In conclusion, our results showed that approximately one third of our patients initially treated with lamivudine, maintained viral suppression >5 years of lamivudine treatment and that add-on adefovir to ongoing lamivudine seems to be a better treatment strategy in patients with lamivudine resistance. However, nowadays, the new generation of inhibitors with the most potent drugs i.e. tenofovir or entecavir demonstrating an optimal resistance profile-high potency and high genetic barrier-, should be used as first-line monotherapies in the treatment of chronic hepatitis B16.
Conflict of interest
The authors declare no competing interests.
Acknowledgement
This work was presented as a poster at the 61st Annual Meeting of the American Association for the Study of the Liver (AASLD), Boston, USA, October 29-November 2, and published as an abstract in Hepatology 2010; 52 (Suppl 4): 519A.
Figure 2. Time to lamivudine resistance with Kaplan-Meier curve.
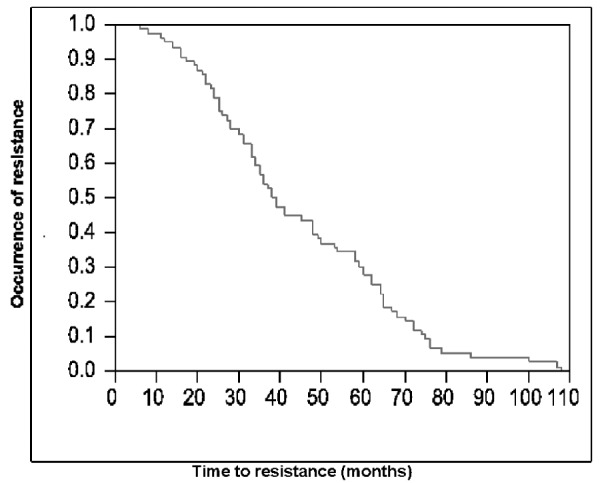
Figure 3. Development of resistance to lamivudine per-every 10 months of follow-up.
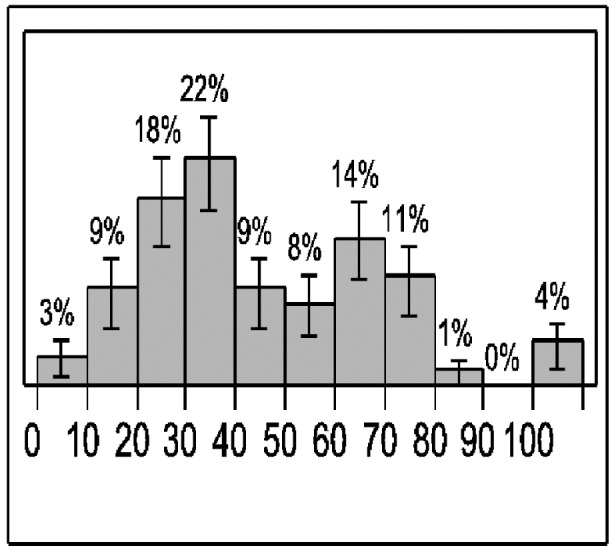
Table 2. Therapeutic strategies in patients with resistance to lamivudine monotherapy.
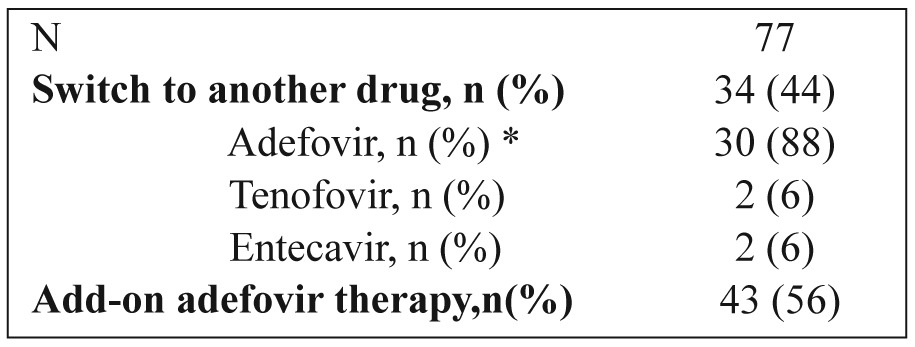
* In 21 patients the switch was preceded by a short (mean 6.4 months) combination with lamivudine.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment and current and emerging prevention and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682–1683. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Vasilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617–624. doi: 10.1053/jhep.2001.27834. [DOI] [PubMed] [Google Scholar]
- 4.Manesis EK. HbeAg-negative hepatitis B: from obscurity to prominence. J Hepatol. 2006;45:343–346. doi: 10.1016/j.jhep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Raptopoulou M, Papatheodoridis G, Antoniou A, Ketikoglou J, Tzourmakliotis D, Vasiliadis T, et al. Epidemiology, course and disease burden of chronic hepatitis B virus infection. HEPNET study for chronic hepatitis B: a multicenter Greek study. J Viral Hepat. 2009;16:195–202. doi: 10.1111/j.1365-2893.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterol. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almaso PL, et al. Italian Association of the Study of the Liver (AISF) Lamivudine Study Group, Italy. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883–891. doi: 10.1002/hep.20381. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687–696. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 9.Keeffe EB, Zeuzem S, Koff RS, Dietrich DT, Esteban-Mur R, Gane EJ, et al. Report of an International workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890–897. doi: 10.1016/j.cgh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Atkins M, Hunt CM, Brown N, Gray F, Sanathanan L, Woessner M, et al. Clinical significance of YMDD mutant hepatitis B virus (HBV) in a large cohort of lamivudine-treated hepatitis B patients. Hepatology. 1998;28:319A. [Google Scholar]
- 11.Zoulim F, Perllo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48:S2–S19. doi: 10.1016/j.jhep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, et al. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121–129. doi: 10.1002/hep.20760. [DOI] [PubMed] [Google Scholar]
- 13.Hadziyiannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAG-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine. A three year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 15.Funk SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 16.EASL clinical practice guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]


