Abstract
Background and aim: The physiological mechanisms regulating zinc homeostasis in humans have been elucidated and described, but the knowledge of zinc status and zinc distribution in the tissues and in the different biological compartments of patients with conservatively treated chronic renal failure (CRF) and on peritoneal dialysis is still insufficient. This investigation examines and compares zinc content in urine, erythrocytes, plasma, and outflow dialysis solution in a group of continuous ambulatory peritoneal dialysis (CAPD) patients, a group of patients with CRF on conservative treatment and in healthy controls.
Material and Methods: Data from the last 6 months of 22 adult hemodialysis patients with a mean age of 61 ± 14 years were analyzed retrospectively. Dialysis vintage, normalized protein catabolic rate (nPCR), serum biochemical parameters, mid arm muscle circumference (MAMC) were determined as mean and standard deviation. Correlations between the variables were computed by coefficient p of Pearson.
Results and conclusion: In patients on CAPD treatment (group 3) compared to healthy controls (group 1) plasma zinc level was diminished (р<0.05), while erythrocyte zinc elevated (р<0.01). The investigation found out difference between plasma, erythrocyte and urine levels of zinc between the patients with chronic renal failure (group 2) on conservative treatment and those treated by CAPD (group 3), which proves, that continuous ambulatory peritoneal dialysis influences redistribution of zinc in human organism "per se".
Keywords: zinc, erythrocytes, plasma, urine, chronic renal failure, continuous ambulatory peritoneal dialysis (CAPD)
Introduction
The ability of the human organism to sustain the balance in its milieu in different and varying environmental conditions is defined as state of homeostasis1. The changes in the levels of zinc absorption and excretion are the crucial mechanism for sustaining zinc homeostasis2-4. In the cases of extremely high or low zinc intake in the organism, adaptive changes in renal excretion of zinc take place3,5,6. Besides this mechanism, cellular and tissue redistribution also occur, and changes in the plasma albumin level as main zinc carrier2,4,7.
In chronic renal failure, derangements in zinc homeostasis may be found due to altered protein metabolism, malabsorption of microelements in the gastrointestinal tract, disturbed renal excretion and expected faulty cellular and tissue redistribution1,6,8,9. Some of the symptoms of chronic renal failure resemble that of zinc deficiency: impaired olfactory function, taste and appetite; growth retardation; impaired wound healing; dry skin; hypogonadism; impaired potency8,10,11. This similarity in the symptoms renders the hypothesis, that some of the symptoms attributed to chronic renal failure are either due to zinc deficiency or are augmented in cases of existing zinc deficiency12-14. Several investigations display decreased plasma levels of zinc in patients with chronic kidney insufficiency conservatively treated or on dialysis1-3,6,7,9,14,15. Some authors find out in similar patient groups zinc level in the erythrocytes in normal range, while others – with elevated levels15-18.
The aim of our investigation was to ascertain the plasma and erythrocyte zinc status in patients with chronic kidney insufficiency conservatively treated or on continuous ambulatory peritoneal (CAPD) and to establish the necessity of zinc supplementation, in case of existing zinc deficiency. In order to avoid erythrocytes` zinc efflux into the plasma, which would negatively affect the correctness of the results, the patients` blood samples were subjected to immediate separation after blood collection.
Material and methods
Thirty six (36) individuals were included in the investigation – 12 healthy controls (group 1), 12 patients with conservatively treated CKD stage 4 (group 2), and 12 patients on CAPD (group 3).
The CAPD patients (group 3) consisted of 6 male and 6 female patients within age limit of 23 to 69 years old, without infectious complications and no oncology diseases, who had started peritoneal dialysis treatment more than three months before the beginning of the investigation. The diagnoses in these patients' kidney diseases included: in 4 cases - chronic pyelonephritis; in 3 cases - chronic glomerulonephritis; in 2 cases - polycystic kidney disease; in 2 cases - gout nephropathy and one patient with hypertensive nephroangiosclerosis. All patients used peritoneal dialysis solutions without zinc and with dextrose content of 1.5%; 2.5% or 4.5% applied in different infusion combinations. All patients carried out 4 exchanges of two litter bags for the 24 hour period.
The patients with conservatively treated chronic renal failure (group 2) also consisted of 6 male and 6 female patients within age limit of 20 to 67 years old, without infectious and oncology diseases. All of them had a creatinine level exceeding 400 mg/l. The diagnoses of primary kidney diseases in this group included: in 4 cases - chronic pyelonephritis; in 3 cases - chronic glomerulonephritis; in 3 cases - polycystic kidney disease; in 1 case - nephrocalcinosis and one patient with hypertensive nephroangiosclerosis.
The control group (group 1) consisted of 6 male and 6 female individuals within age limit of 20 to 65 years old. All these participants were clinically healthy and none of them was subjected to dietary restrictions during the period of the investigation.
All samples were taken after overnight fast between 8 and 10 in the morning. The participants in the study were instructed to collect 24 hour total urine, which was measured as quantity and from which, samples were taken in zinc-free containers for measurements of zinc and total protein content. The patients on CAPD were instructed to collect all the bags with effluent dialysis solutions for the 24 hour periods, which were also measured quantitatively and in samples taken in zinc-free containers the zinc level was investigated. Five ml of blood samples in zinc-free containers were taken from every participant in the investigation and immediately centrifuged to prevent the zinc efflux due to haemolysis from the cells into the plasma, or influx of zinc into the erythrocytes.
The albumin, total protein and creatinine were routinely tested with Hitachi-912 Autoanalyser. The zinc content of the erythrocytes and in plasma, urine and effluent peritoneal solution was ascertained by atomic absorption spectrophotometry with Perkin Elmer-5100 PC. Control tests of all solutions and laboratory vials were analyzed for eventual zinc contamination, but all samples proved negative.
All patients and healthy controls signed a written informed consent before the investigation and the study was approved by the Ethical Committee of our hospital.
Statistical analysis:
Values are expressed as mean ± SD. Statistical comparisons between the groups were carried out using Mann Whitney U tests. A p value < 0.05 was set as statistical significant.
Results
The blood values for total protein, albumin, creatinine, hemoglobin and erythrocytes in the investigated groups are exhibited in Table 1.
Table 1. Blood parameters comparison between groups 1, 2, 3.
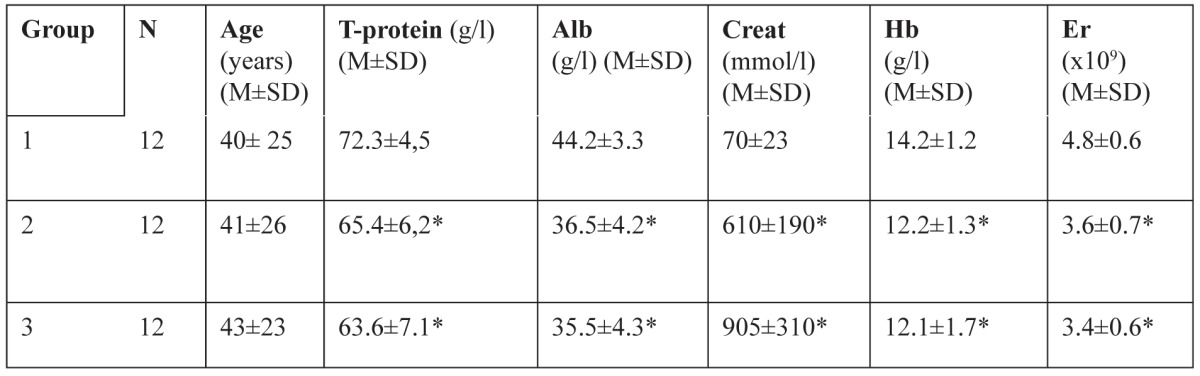
Legend of Table 1: number (N), age, and blood levels of T-proteins, albumin (Alb), creatinine (Creat), hemoglobine (Hb) and erythrocytes (Er) in group of healthy controls (1), group of patients with chronic renal failure (CRF) - stage 4 chronic kidney diseases (CKD) (2) and group of patients on continuous ambulatory peritoneal dialysis (CAPD) (3). * Significant change from normal control values (р<0.01).
The total protein, albumin, hemoglobin and erythrocytes count were decreased in the patients` groups with conservatively treated chronic renal failure and on continuous ambulatory peritoneal dialysis treatment (group 2 and 3) with the average values statistically significantly lower than in the control group 1. The creatinine value was within normal range in the control group and significantly elevated in the other groups.
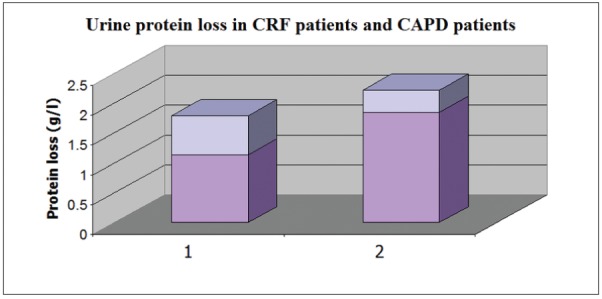
Urine protein losses were more pronounced in patients with CKD on conservative treatment, compared to those on CAPD treatment (1.1461.85 vs. 0.6560.37 g/l; p<0.01) (Figure 1).
Figure 1. Urine protein loss in CRF patients (1) and CAPD patients (2). Values represents Mean ± Standard Deviation.
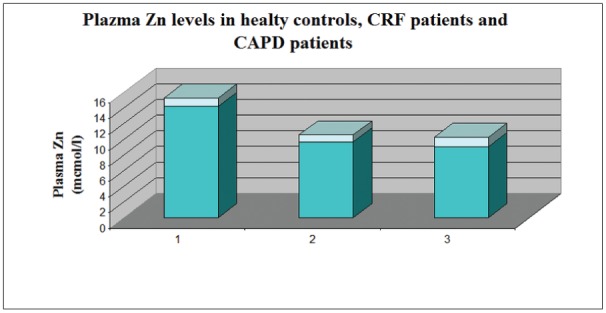
The plasma zinc content in healthy controls (group 1) exhibits normal values (14.2±1.1 mmol/l) while it showed moderately decreased values in CRF patients on conservative treatment (group2) (9.7±0.9mmol/l ) and on CAPD treatment (group 3) (9.1±1.2mmol/l ) (Figure 2).
Figure 2. Plasma Zn levels in the control group (1), in CRF patients' group (2) and CAPD patients' group (3). Values represents Mean ± Standard Deviation.
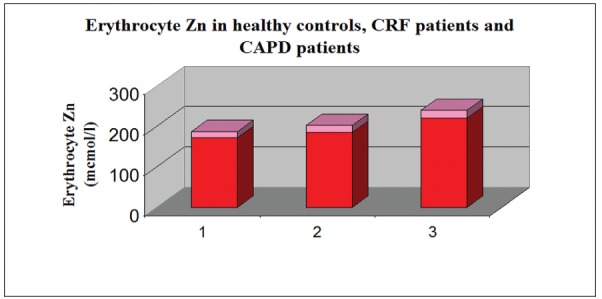
The erythrocyte zinc was in normal ranges in healthy controls (172±15 mmol/l), while in the other two groups of patients (2nd and 3) it was significantly elevated, compared to group 1 (р<0.01). (Figure 3).
Figure 3. Erythrocyte Zn levels in the controls (group 1), CRF patients (group 2) and CAPD patients (group 3). Values represents Mean ± Standard Deviation.
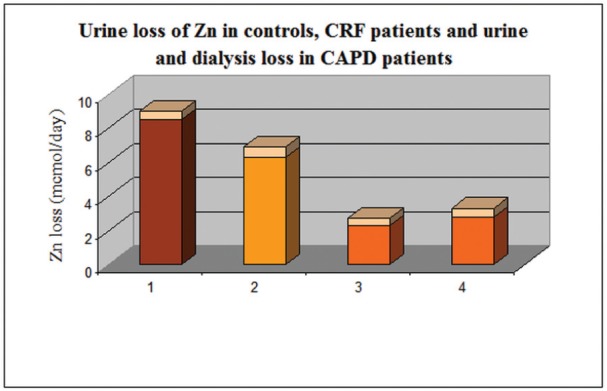
The urine zinc content was within normal values in the three investigated groups (< 10 mmol/day). The patients on CAPD excreted significantly lower quantities compared to the other two groups. In this group, the daily losses of zinc with the effluent peritoneal solutions (2.8±0.5 mmol/day), were similar to the urine losses (2.3±0.4 mmol/day). The total excretion of zinc (urine plus peritoneal dialysis solution) - 5.1±0.4mmol/day - was lower compared to the urine losses in the two other groups – healthy controls (8.5±0.5 mmol/day) and conservatively treated chronic renal failure (6.3±0.6 mmol/day). Urine and dialysate excretion of zinc in the three groups are presented on Figure 4.
Figure 4. Urine loss of Zn in control group(1), CRF group(2), CAPD group(3) and effluent dialysis Zn loss in CAPD patients(4).
Discussion
The microelements play an important role in the regulation of vital processes1,2,4,6. The patients with chronic kidney diseases in the stages of chronic renal failure, on conservative and dialysis treatment have a complex of metabolic abnormalities leading to serious complications1,6,7,9,18. Most of the authors stress on the fact, that uraemia "per se" leads to an imbalance affecting the microelements in human body and the dialysis treatment is incapable to secure a full restoration of the required balance1,5,7,10,11,15. The deranged homeostasis of microelements, among which that of zinc, can be potentially a factor, which triggers some of the uraemic complications. Zinc is an essential component in more than 120 zinc-dependant enzymes and hence, the symptoms of zinc deficiency are complex and non-specific. They can simulate deficiency of essential amino and lipid acids, vitamins and can also resemble symptoms usually attributed to chronic renal insufficiency6,8,9,14,17. In the existing literature on this topic, the predominant part of the authors verify decreased plasma levels of zinc in patients with chronic renal failure on conservative or dialysis treatment1,2,5,7,9,12,15. Concerning the cellular zinc content, conflicting data exists – normal or mildly diminished content in some investigations, while other authors find out elevated erythrocyte and lymphocyte zinc content1,6,9,14-16. The findings in some investigations hypothesize zinc deficiency, while in others the explanations are, that there is redistribution of zinc in the biologic compartments of uraemic patients1,2,5,15,18.
We consider, taking into account the results of our investigation, that the patients` groups with conservatively treated CKD and on CAPD treatment exhibit a moderate zinc deficiency with compensatory erythrocyte zinc overloading. This reasonable assumption is demonstrated by the significantly lower zinc plasma levels in the groups with conservatively treated CKD and on CAPD treatment compared to healthy controls (Figure 2); (р<0.05), and mild to moderate elevation of erythrocyte zinc values in both CKD groups (2 and 3), reaching significant difference in the CAPD patients from the values of the control group (Figure 3) (р<0.01). Although existing proteinuria in both CKD groups is found out, we do not establish increased urinary zinc loss. On the contrary there is a mild (in conservatively treated patients) to significant (in CAPD patients) decreased zinc excretion in urine (Figure 1). This finding is most probably due to the low level of proteinuria in the investigated groups on one side and on the other - to the preserved to some extent adaptive change of renal excretion, although many other renal functions are lost in the state of chronic renal insufficiency. On the background of the discussed existing zinc sparing mechanisms in both CRF groups, the ascertained zinc deficiency in the patient group with CRF on conservative treatment is most probably due to some protein-calorie restrictions, which had been therapeutically justified. In the CAPD group, since these patients are not exposed to dietetic restrictions of foods with high zinc content, zinc deficiency may be attributed to the long time existing uraemic intoxication leading to decreased appetite and intestinal mal-absorption of zinc. (The last two mentioned factors – intoxication and mal-absorption, although to a smaller extent exist in the conservatively treated group 2 too). In relation to the zinc loss in CAPD, we can not state an explicit attitude, since the effluent peritoneal solution remain in the bags for a long period of time before the zinc content is tested. We may hypothesize, that there is a potential opportunity, the chemical material used for the production of the bags, to absorb on its` surface zinc from the effluent solution and hence to account for the findings of low quantity zinc losses in effluent peritoneal dialysate.
In conclusion, we can summarize, that uremia and peritoneal dialysis procedure by themselves create conditions for the development of abnormally low body zinc content, as well as its` redistribution in tissues and fluids. It should be controlled by assessments of zinc content in the before mentioned body compartments and adequately supplemented, with the aim to prevent clinical manifestations of zinc deficiency, some of which are morbid and rather dramatic.
Conflict of interest statement.
There is no conflict of interests.
References
- 1.Hsieh YY, Shen WS, Lee LY, Wu TL, Ning HC, Sun CF. Long-term changes in trace elements in patients undergoing chronic hemodialysis. Biol Trace Elem Res. 2006;109:115–121. doi: 10.1385/BTER:109:2:115. [DOI] [PubMed] [Google Scholar]
- 2.Krachler M, Scharfetter H, Wirnsberger GH. Kinetics of the metal cations magnesium, calcium, copper, zinc, strontium, barium, and lead in chronic hemodialysis patients. Clin Nephrol. 2000;54:35–44. [PubMed] [Google Scholar]
- 3.Neiva TJ, Benedetti AL, Tanaka SM, Santos JI, D'Amico EA. Determination of serum aluminum, platelet aggregation and lipid peroxidation in hemodialyzed patients. Braz J Med Biol Res. 2002;35:345–350. doi: 10.1590/s0100-879x2002000300009. [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R, Cornelis R, Dhondt A, Lameire N. The role of trace elements in uraemic toxicity. Nephrol Dial Transplant. 2002;17:2–8. doi: 10.1093/ndt/17.suppl_2.2. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kopple JD. Trace elements and vitamins in maintenance dialysis patients. Adv Ren Replace Ther. 2003;10:170–182. doi: 10.1053/j.arrt.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Richard MJ, Arnaud J, Jurkovitz C, Hachache T, Meftahi H, Laporte F, et al. Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991;57:10–15. doi: 10.1159/000186208. [DOI] [PubMed] [Google Scholar]
- 7.Krachler M, Wirnsberger G, Irgolic KJ. Trace element status of hemodialyzed patients. Biol Trace Elem Res. 1997;58:209–221. doi: 10.1007/BF02917472. [DOI] [PubMed] [Google Scholar]
- 8.Zima T, Tesar V, Mestek O, Nemecek K. Trace elements in end-stage renal disease. 1. Methodological aspects and the influence of water treatment and dialysis equipment. Blood Purif. 1999;17:182–186. doi: 10.1159/000014394. [DOI] [PubMed] [Google Scholar]
- 9.Zima T, Tesar V, Mestek O, Nemecek K. Trace elements in end-stage renal disease. 2. Clinical implication of trace elements. Blood Purif. 1999;17:187–198. doi: 10.1159/000014395. [DOI] [PubMed] [Google Scholar]
- 10.Krachler M, Scharfetter H, Wirnsberger G. Exchange of alkali trace elements in hemodialysis patients:a comparison with Na(+) and K(+) Nephron. 1999;83:226–236. doi: 10.1159/000045515. [DOI] [PubMed] [Google Scholar]
- 11.Krachler M, Wirnsberger GH. Long-Term Changes of Plasma Trace Element Concentrations in Chronic Hemodialysis Patients. Blood Purif. 2000;18:138–143. doi: 10.1159/000014437. [DOI] [PubMed] [Google Scholar]
- 12.Kazi TG, Jalbani N, Kazi N, Jamali MK, Arain MB, Afridi HI, et al. Evaluation of toxic metals in blood and urine samples of chronic renal failure patients, before and after dialysis. Ren Fail. 2008;30:737–745. doi: 10.1080/08860220802212999. [DOI] [PubMed] [Google Scholar]
- 13.Cohen N, Golik A. Zinc balance and medications commonly used in the management of heart failure. Heart Fail Rev. 2006;11:19–24. doi: 10.1007/s10741-006-9189-1. [DOI] [PubMed] [Google Scholar]
- 14.Guo CH, Wang CL, Chen PC, Yang TC. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in pacients undergoing either hemodialysis or peritoneal dialysis. Perit Dial Int. 2011;31:583–591. doi: 10.3747/pdi.2009.00225. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima T, Horike H, Fujiki S, Kitada S, Sasaki T, Kashihara N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Ther Apher Dial. 2009;13:213–219. doi: 10.1111/j.1744-9987.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 16.Lowe NM, Fekete K, Desci T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. 2009;89:2040S–2051S. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 17.Puchades Montesa MJ, González Rico MA, Solís Salguero MA, Torregrosa Maicas I, Tormos Muñoz MC, Saez Tormo G, et al. [Study of oxidative stress in advanced kidney disease] Nefrol. 2009;29:464–473. doi: 10.3265/Nefrologia.2009.29.5.5493.en.full. [DOI] [PubMed] [Google Scholar]
- 18.Noleto Magalhães RC, Guedes Borges de Araujo C, Batista de Sousa Lima V, Machado Moita Neto J, do Nascimento Nogueira N, do Nascimento Marreiro D. Nutritional status of zinc and activity superoxide dismutase in chronic renal patients undergoing hemodialysis. Nutr Hosp. 2011;26:1456–1461. doi: 10.1590/S0212-16112011000600037. [DOI] [PubMed] [Google Scholar]


