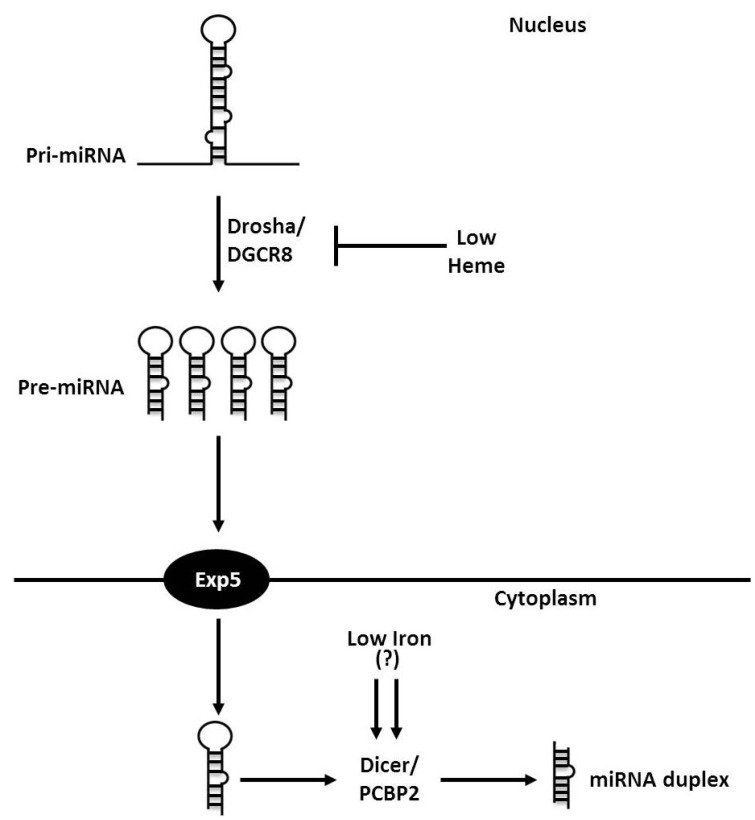
Figure 1.
Relationship between cellular iron and miRNA processing. Following their transcription, primary miRNA (pri-miRNA) are cleaved at the stem of the hairpin structure by the RNase II-type protein Drosha and its cofactor DGCR8, a heme-binding protein. Heme-free DGCR8 is less active than heme-bound DGCR8 suggesting that cellular iron status may affect the rate and efficiency of pri-miRNA processing. The product of Drosha/DGCR8 processing is an ~70 nucleotide long precursor miRNA (pre-miRNA) that is exported out of the nucleus into the cytoplasm by the nuclear export factor exportin 5 (Exp5) through the recognition of a short 3′-overhang on the pre-miRNA. Upon entry into the cytoplasm, the RNase III-like enzyme Dicer catalyzes the second processing step of “dicing” the pre-miRNA to produce an ~22 nucleotide long miRNA duplex. Preliminary evidence suggests that iron also regulates the processing of pre-miRNA via the iron-dependent regulation of Dicer activity through its association with poly(rC)-binding protein 2 (Pcbp2), wherein the removal of cytosolic iron, but not heme-iron, enhances pre-miRNA processing. Following cleavage by Dicer, the miRNA duplex is available to be assembled into the RISC to participate in RNA silencing of target mRNA.