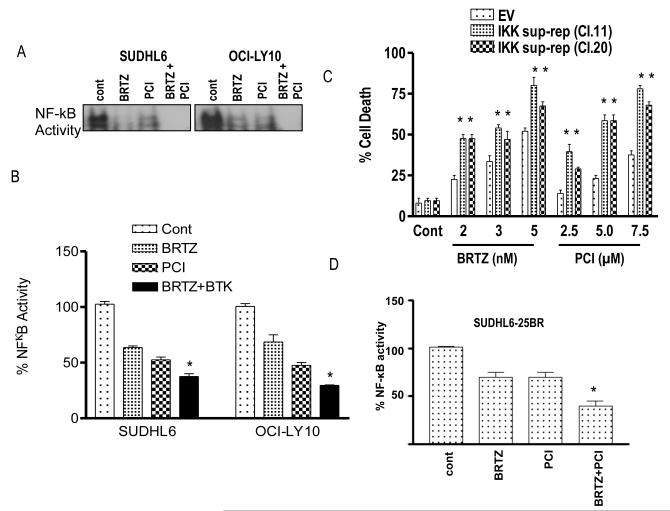
Figure 5. Evidence for the contribution of inactivation of NF-κB to PCI-32765/bortezomib synergism.
(A) SUDHL 6 and OCI-LY10 - DLBCL cells were treated with bortezomib (3.0-8.0 nM) ± PCI (6-7.5 μM) and for 24 h. Nuclear proteins were extracted using a nuclear extract kit (Active Motif) and then subjected to electrophoretic mobility shift assay (EMSA) gel shift assays to assess NF-κB DNA binding as described in Methods. (B) Using the same nuclear proteins, NF-κB activity was determined using an enzyme-linked immunosorbent assay (ELISA) TransAM NF-κB p65 Transcription Factor Assay Kit (Active Motif), as described in Methods. (C) SUDHL16-IKK super repressor cell (sup-rep) with empty vector control was treated with the indicated concentration of either bortezomib or PCI alone for 48 h and cell death was measured by 7AAD staining. (D) SUDHL 6-25BR cells were treated with bortezomib (15 nM) ± PCI (7.5 μM) for 24 h. Nuclear proteins were extracted using a nuclear extract kit (Active Motif) and NF-κB activity was determined using an ELISA TransAM NF-κB p65 Transcription Factor Assay Kit (Active Motif), as described in Methods. Values represent the means ± standard deviation of triplicate determinations for 3 separate experiments. For B & D * = significantly less than values for bortezomib or PCI alone; P < 0.05, For C ** = significantly greater than values for bortezomib or PCI alone than empty vector control; P < 0.05. cont, control; BRTZ, bortezomib; PCI, PCI-32765.