Figure 2.
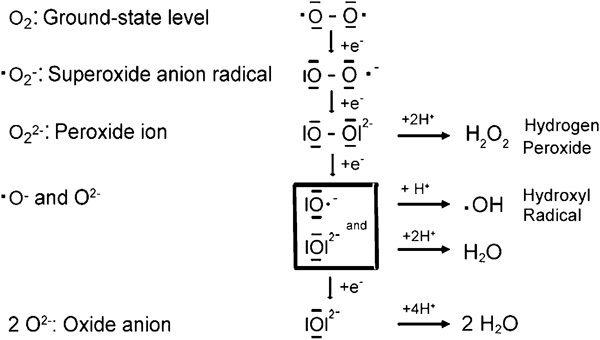
Oxidation forms of oxygen. If molecular oxygen, which is a diradical with two unpaired electrons, is reduced, it acquires four electrons and water (H2O) is formed. The dashes around the oxygen (O) represent paired, the points represent unpaired electrons.
