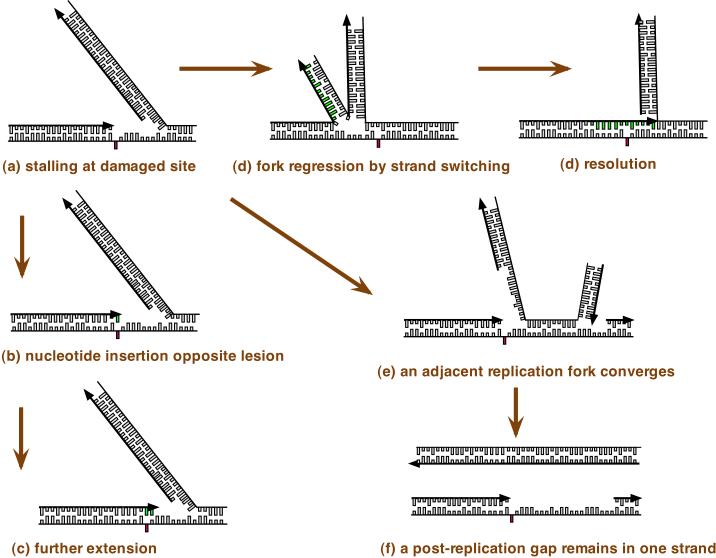
Figure 2. Strategies for translesion DNA synthesis.
Some types of DNA damage, if not repaired, will block the progression of a DNA replication fork. When a site of DNA damage on the leading strand is encountered by the DNA replication machinery and this prevents normal base pairing (red rectangle), replication is blocked. The lagging strand may continue replication, but the leading strand on which the replication machinery is blocked is fragile. Replication on the two strands can uncouple and dissociation of the DNA replication machinery causes ‘collapse’ of the DNA replication fork, eventually leading to a DNA break (Figure 1). Several possible strategies to overcome this block to replication may be activated. One strategy (part a) is to carry out translesion DNA synthesis (TLS) by successive steps. The replication machinery switches to a specialized DNA polymerase for insertion of a base. This step is potentially mutagenic because the wrong base will sometimes be incorporated. A switch to a second specialized DNA polymerase may take place to extend the nonstandard terminus opposite the damage, and finally there is a switch to a replicative DNA polymerase (POLε or POLδ). DNA polymerase switching is facilitated by post-translational modifications of DNA polymerases and their accessory factors, as summarized in the text and reviewed in depth elsewhere 1, 3, 98, 99. A second strategy (b) is DNA replication fork regression. Here, the blocked leading strand switches templates and begins to copy the already-replicated lagging strand. The newly-replicated bases are shown in green. The regressed fork resembles a four-way junction that can be processed by homologous recombination enzymes and resolved. This pathway avoids errors, as it makes use of genetic information from the undamaged strand. A third strategy is illustrated in part c. If the replication fork remains stalled for long enough, an adjacent replication fork will converge with it. This allows one strand to replicate fully, while one strand will contain a gap. This gap will then remain through to late S phase or G2 phase of the cell cycle. The gap is then filled by DNA synthesis. During gap filling, two different specialized DNA polymerases may also be needed to accomplish synthesis across from a lesion, for insertion and extension, and this is potentially mutagenic. Gaps could also conceivably arise by re-initiation of DNA synthesis on the other side of a DNA adduct. Arrows indicate the direction of DNA replication, which is 5’ to 3’ with respect to the deoxyribose sugar-phosphate.