In a large human immunodeficiency virus–hepatitis C virus coinfection cohort, we found no evidence that marijuana smoking accelerated progression to significant liver fibrosis, cirrhosis, or end-stage liver disease. Previous studies reporting an association may have been biased by reverse causation due to self-medication.
Keywords: HIV, HCV, cannabis, liver disease, cohort study
Abstract
Background. Marijuana smoking is common and believed to relieve many symptoms, but daily use has been associated with liver fibrosis in cross-sectional studies. We aimed to estimate the effect of marijuana smoking on liver disease progression in a Canadian prospective multicenter cohort of human immunodeficiency virus/hepatitis C virus (HIV/HCV) coinfected persons.
Methods. Data were analyzed for 690 HCV polymerase chain reaction positive (PCR-positive) individuals without significant fibrosis or end-stage liver disease (ESLD) at baseline. Time-updated Cox Proportional Hazards models were used to assess the association between the average number of joints smoked/week and progression to significant liver fibrosis (APRI ≥ 1.5), cirrhosis (APRI ≥ 2) or ESLD.
Results At baseline, 53% had smoked marijuana in the past 6 months, consuming a median of 7 joints/week (IQR, 1–21); 40% smoked daily. There was no evidence that marijuana smoking accelerates progression to significant liver fibrosis (APRI ≥ 1.5) or cirrhosis (APRI ≥ 2; hazard ratio [HR]: 1.02 [0.93–1.12] and 0.99 [0.88–1.12], respectively). Each 10 additional joints/week smoked slightly increased the risk of progression to a clinical diagnosis of cirrhosis and ESLD combined (HR, 1.13 [1.01–1.28]). However, when exposure was lagged to 6–12 months before the diagnosis, marijuana was no longer associated with clinical disease progression (HR, 1.10 [0.95–1.26]).
Conclusions In this prospective analysis we found no evidence for an association between marijuana smoking and significant liver fibrosis progression in HIV/HCV coinfection. A slight increase in the hazard of cirrhosis and ESLD with higher intensity of marijuana smoking was attenuated after lagging marijuana exposure, suggesting that reverse causation due to self-medication could explain previous results.
In developed countries, over 30% of persons infected with human immunodeficiency virus (HIV) are coinfected with hepatitis C virus (HCV) [1], and HCV has been shown to progress more rapidly in the presence of HIV infection [2]. Liver disease is an important and growing cause of morbidity and mortality in coinfected patients [3, 4]. While impaired immunity due to HIV infection may partly explain more rapid fibrosis progression, coinfected patients experience a number of other potentially hepatotoxic exposures that could contribute to liver disease such as illicit drug use.
Marijuana is widely used in Canada: in a 2005 survey, 44% of Canadians reported cannabis use in their lifetime, and 14% reported use in the past year [5]. In a study of medication and alternative therapy use among 104 HIV patients in Ontario, 43% of patients self-reported marijuana use in the past year, and 29% reported a medicinal use [6].
The literature regarding the effects of cannabis on liver diseases is conflicting. Cell culture and animal model studies support that cannabinoids could have a therapeutic effect on liver injury and fibrosis progression [7–12]. However, 3 cross-sectional studies in patients with chronic HCV suggest that daily cannabis use is associated with fibrosis and steatosis [13–15]. A small cohort study of 58 HIV/AIDS patients reported no statistically significant change in liver enzymes in dronabinol and/or marijuana users over the span of 1 year [16]. There have been no large prospective studies of the effect of cannabis on liver fibrosis progression. Despite this, there is a general acceptance that cannabis use negatively affects liver fibrosis.
This study aimed to estimate the effect of marijuana smoking on liver disease progression longitudinally in HIV-HCV coinfected individuals.
METHODS
Cohort Design and Study Population
The Canadian Coinfection Cohort study is a multicenter longitudinal study of HIV-HCV coinfected individuals from 17 HIV clinics across Canada. The eligibility criteria are: (1) over 16 years old; (2) documented HIV infection (HIV positive by enzyme-linked immunosorbant assay with Western blot confirmation); and (3) evidence of HCV infection (HCV seropositive by enzyme-linked immunosorbant assay with recombinant immunoblot assay II or enzyme immunoassay confirmation, or if serologically false negative, HCV RNA positive).
After informed consent, each participant underwent an initial evaluation followed by study visits approximately every 6 months. At each visit, sociodemographic and behavioral information were self-reported in questionnaires; medical treatments and diagnoses were collected by research personnel and laboratory analyses, including aspartate aminotransferase (AST) and platelet count, were performed. Details of the cohort are presented elsewhere [17]. As of 1 July 2012, 1118 patients had been recruited and followed for a median of 31.8 months (interquartile range [IQR], 6.0–36.9). A subcohort was defined for this study and included all coinfected patients with HCV replication (plasma HCV RNA reverse transcription polymerase chain reaction [RT-PCR], Roche COBAS Amplicor), without significant fibrosis (AST-to-platelet ratio [APRI] score <1.5), radiological or histological diagnosis of cirrhosis and/or end-stage liver disease (ESLD) at baseline.
Marijuana Use
At each study visit, participants were asked to report their marijuana use since the last interview. Marijuana smokers also reported how often they smoked (occasionally/not every week, regularly/1–2 days per week, regularly/3–6 days per week, every day) and the number of joints consumed on the days they smoked. The average number of joints smoked per week for each interval was calculated by multiplying the number of joints reported by the mean number of days in the frequency interval reported. Participants were also asked for what reason they smoked marijuana. No information was collected on ingestion of marijuana.
Significant Liver Fibrosis, Cirrhosis and End-stage Liver Diseases
At each visit, the APRI was calculated as: APRI = 100(AST/upper limit of normal)/platelet count (109/L) to predict significant fibrosis and cirrhosis. An APRI score ≥1.5 was used to indicate significant liver fibrosis (equivalent to a score ≥2 on the Metavir scale), and a score ≥2 was used to indicate cirrhosis (equivalent to a score of 4 on the Metavir scale) [18]. Clinical cirrhosis was determined radiologically or by liver biopsy. ESLD was defined as a diagnosis of ascites, portal hypertension, spontaneous bacterial peritonitis, encephalopathy, oesophageal varices, or hepatocellular carcinoma collected using dedicated case report forms and validated centrally.
Statistical Analyses
Participants were censored at initiation of HCV treatment because treatment can affect the AST and platelet counts, thus impacting the measurement of APRI score, and when curative, reduces risk of fibrosis progression and ESLD. Time-dependent Cox proportional hazards models were used to assess the presence of an association between the number of joints smoked per week and progression to significant liver fibrosis, cirrhosis or ESLD. The average number of joints smoked per week in the 6 months preceding the study visit and a binary indicator of marijuana smoking were updated at each study visit. All models were adjusted for baseline APRI score, age, duration of HCV infection, sex, and income and for time-updated CD4 cell count, HIV viral load and antiretroviral therapy (ART) use at the preceding visit, and alcohol abuse and injection drug use in the 6 months preceding the visit. Duration of HCV infection was based on the date of HCV seroconversion, if known, or year of first injection drug use (IDU) or blood product exposure as a proxy of HCV infection [19]. Income was treated as a dichotomous variable, using a yearly income of 24 000 CAD as the cutoff based on low-income cutoffs calculated by Statistics Canada [20]. Alcohol abuse was defined as drinking at least 3 alcoholic drinks on a typical day when drinking and having ≥6 drinks on one occasion more than once per month.
Reverse causation bias may occur when patients modify their behavior due to illness, for example, start or increase their marijuana consumption to alleviate symptoms related to advancing liver disease. Thus, when exposure and outcome are ascertained concurrently, it could appear that marijuana causes significant fibrosis when in fact fibrosis was present before marijuana exposure occurred. We therefore repeated the analyses using marijuana smoking exposures 6–12 months before outcome assessment in order to reduce the possibility of reverse causation by ensuring temporality of the exposure-outcome relationship is preserved.
We investigated the presence of a dose-response relationship between the number of joints smoked per week and the natural logarithm of the APRI score with a linear spline regression model, introducing flexibility to linear regression by allowing the slope of the curve to change at the prespecified knots. The location of the knots was selected a priori based on meaningful cutoff at 7 joints/week (eg, regular, low dose use) and 21 joints/week. This model was adjusted for baseline age, sex, and income, and for updated alcohol use, IDU, ART use, CD4 cell count, HIV RNA, and duration of HCV infection.
RESULTS
A total of 690 participants conformed to the eligibility criteria of this study, contributing 1875.3 person-years of follow-up, with a median follow-up time of 2.7 years (IQR, 0.8–3.8). Selection of the study population is represented in Figure 1. The majority of participants were male, with low income, and the median age at baseline was 44 years. The majority of the participants had undetectable HIV viral loads; the median CD4 cell count was 400 and 38% used injection drugs at baseline. About half the participants used alcohol, among whom 53 (15%) reported alcohol abuse at baseline. The baseline and updated characteristics of the study population are presented in Table 1.
Figure 1.
Study population flow chart. Abbreviations: ESLD, end-stage liver disease; HCV, hepatitis C virus.
Table 1.
Baseline and Updated Characteristics of the Study Population
| Characteristics | Baseline | Follow-upa |
|---|---|---|
| No. (persons or person-visits) | 690 | 3112 |
| Follow-up time (months), median (IQR) | ( … ) | 32.2 (10.1–45.6) |
| Age (years), median (IQR) | 43.9 (38.4–49.2) | ( … ) |
| Male, No. (%) | 503 (72.9) | ( … ) |
| Yearly income <24 000 CAD, No. (%) | 583 (84.5) | ( … ) |
| Homeless, No. (%) | 33 (4.8) | ( … ) |
| Alcohol use in past 6 mo, No. (%) | 348 (50.4) | 1724 (55.4) |
| Alcohol abuse in past 6 mo (among alcohol users), No. (%) | 53 (15.2) | 253 (14.7) |
| Used injection drugs in past 6 mo, No. (%) | 263 (38.1) | 1064 (34.2) |
| Duration of HCV infection (years), median (IQR) | 18.0 (10.4–24.5) | ( … ) |
| Duration of HIV infection (years), median (IQR) | 10.3 (5.6–15.6) | ( … ) |
| CD4 cell count, median (IQR) | 400 (270–570) | 420 (280–598) |
| CD4 cell count <200 cells/mm3, No. (%) | 99 (14.3) | 394 (12.7) |
| Undetectable HIV viral load (<50), No. (%) | 375 (54.3) | 2018 (64.8) |
| BMI, median (IQR) | 24.0 (21.2–26.8) | 23.5 (21.0–26.8) |
| Non-fasting glucose (mmol/L), median (IQR) | 5.1 (4.6–5.8) | 5.1 (4.6–5.8) |
| Diabetes, No. (%) | 24 (3.5) | 39 (5.6) |
| APRI score, median (IQR) | 0.52 (0.37–0.79) | 0.55 (0.37–0.90) |
| First occurrence of APRI ≥ 1.5, No. (%) | ( … ) | 132 (19.1) |
| First occurrence of APRI ≥ 2, No. (%) | ( … ) | 102 (14.8) |
| First occurrence of cirrhosis, No. (%) | ( … ) | 8 (1.2) |
| First occurrence of end-stage liver disease, No. (%) | ( … ) | 11 (1.6) |
| First occurrence of cirrhosis or end-stage liver disease, No. (%) | ( … ) | 16 (2.3) |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio; BMI, body mass index; CAD, Canadian dollars; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
a The numbers in this column represent the number and proportion of intervals during follow-up in which each characteristic was reported (No. [%]), or the distribution of the characteristic for all intervals of follow-up (median (IQR)).
Table 2 summarizes marijuana smoking behaviors in the study population at baseline and over follow-up. Over half of the participants reported smoking marijuana in the past 6 months (median, 7 joints/week). About 40% of marijuana users reported smoking daily. A little over 40% of participants who reported marijuana smoking used it for symptom relief at baseline, and this proportion increased to half during follow-up.
Table 2.
Marijuana Smoking Behaviors of the Study Population
| Marijuana Smoking Characteristic | Baseline (n = 690 persons) | Follow-up (n = 3112 person-visits) |
|---|---|---|
| Smoked in past 6 mo/since last interview, No. (%) | 367 (53.2) | 1654 (53.1) |
| Smoking frequency, No. (%; among smokers) | ||
| Occasionally, not every week | 119 (32.4) | 513 (31.0) |
| Regularly, 1–2 d/wk | 50 (13.6) | 203 (12.3) |
| Regularly, 3–6 d/wk | 50 (13.6) | 232 (14.0) |
| Everyday | 145 (39.5) | 685 (41.4) |
| Missing | 3 (0.8) | 21 (1.3) |
| No. of joints/week, median (IQR) | 7 (1–21) | 7 (1–27) |
| Main reason for smokinga, No. (%) | ||
| To relieve symptoms | 150 (40.9) | 838 (50.7) |
| To increase appetite | 152 (41.4) | 827 (50.0) |
| Recreational purposes | 167 (45.5) | 776 (46.9) |
| Sleep | 1 (0.3) | 1 (0.1) |
Abbreviation: IQR, interquartile range.
a Categories are not mutually exclusive.
Progression to Liver Diseases
Over the course of follow-up, 132 persons (19.1%) reached an APRI score of 1.5, 102 (14.8%) reached a score of 2, 8 developed cirrhosis alone (1.2%), and another 11 (1.6%) developed end-stage liver disease. The incidence rate for progression to an APRI ≥ 1.5 was 39.2 per 1000 person-visits; to an APRI ≥ 2, 29.2 per 1000 person-visits; to a clinical diagnosis of cirrhosis, 2.1 per 1000 person-visits; and to ESLD, 2.9 per 1000 person-visits. There were no differences in the crude rates between marijuana users and nonusers for any of the outcomes assessed.
Tables 3 and 4 present the results of the multivariate models for the association between marijuana smoking and progression to liver diseases. No significant association could be found between marijuana use and development of significant fibrosis or cirrhosis measured with the APRI score. Lagging the exposure variable by one visit had no impact on these conclusions. Neither current nor lagged marijuana smoking accelerated progression to ESLD.
Table 3.
Effect of Marijuana Smoking on Progression of Liver Diseases
| Outcome | Model | Hazard Ratio (95% CI) |
|---|---|---|
| APRI ≥ 1.5 | 10 joints/wk, currenta | 1.02 (.93, 1.12) |
| Lagged exposureb | 0.95 (.85, 1.07) | |
| APRI ≥ 2 | Current exposurea | 0.99 (.88, 1.12) |
| Lagged exposureb | 0.96 (.85, 1.10) | |
| Cirrhosis | Current exposurea | 1.33 (1.09, 1.62) |
| Lagged exposureb | 1.12 (.94, 1.34) | |
| ESLD | Current exposurea | 1.08 (.90, 1.28) |
| Lagged exposureb | 1.07 (.85, 1.34) | |
| Cirrhosis or ESLD | 10 joints/wk, currenta | 1.13 (1.01, 1.28) |
| Lagged exposureb | 1.10 (.95, 1.26) |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio; CI, confidence interval; ESLD, end-stage liver disease.
a Current exposure models report on the effect associated with an increase of 10 joints per week in the past 6 mo. Models are adjusted for baseline: age, duration of hepatitis C virus infection, sex, and income, time-updated alcohol and injection drug use in the 6 mo preceding the visit, CD4 cell count, human immunodeficiency virus viral load, and antiretroviral therapy use at the preceding visit and binary indicator of marijuana smoking.
b Lagged exposure models report on the effect associated with an increase of 10 joints per week in the past 6–12 mo prior to the current follow-up visit. Models adjusted for same variables, with the exception of binary indicator of marijuana smoking relating to 6–12 mo before outcome assessment.
Table 4.
Full Models for the Effect of Current Marijuana Smoking on Progression to Significant Liver Fibrosis (Aspartate Aminotransferase-to-Platelet Ratio Score ≥1.5) and Cirrhosis or End-stage Liver Disease
| Outcome | Model | Hazard Ratio (95% CI) |
|---|---|---|
| APRI ≥ 1.5 | 10 joints/wk, current | 1.02 (.93, 1.12) |
| Marijuana use, current | 0.78 (.51–1.20) | |
| Baseline | ||
| APRI score | 6.12 (3.46–10.83) | |
| Age (per 5 y) | 1.00 (.87–1.14) | |
| Duration of HCV infection (per 5 y) | 1.06 (.95–1.18) | |
| Female | 1.50 (.99–2.25) | |
| Income <24 000 CAD | 0.92 (.49–1.75) | |
| Time-updated | ||
| Alcohol abuse | 1.49 (.84–2.62) | |
| Injection drug use | 1.12 (.75–1.69) | |
| ART use | 1.00 (.56–1.80) | |
| CD4 count (per 100 cell) | 0.98 (.91–1.06) | |
| HIV RNA (log copies/mL) | 1.03 (.94–1.12) | |
| Cirrhosis or ESLD | 10 joints/wk, current | 1.13 (1.01, 1.28) |
| Marijuana use, current | 0.72 (.20–2.55) | |
| Baseline | ||
| APRI score | 2.41 (.43–13.41) | |
| Age (per 5 y) | 1.34 (.91–1.98) | |
| Duration of HCV infection (per 5 y) | 1.01 (.77–1.34) | |
| Female | 1.06 (.27–4.15) | |
| Income <24 000CAD | 0.49 (.12–2.03) | |
| Time-updated | ||
| Alcohol abuse | 1.08 (.13–8.65) | |
| Injection drug use | 0.42 (.09–2.04) | |
| ART use | ( … ) | |
| CD4 count (per 100 cell) | 0.93 (.72–1.19) | |
| HIV RNA (log copies/ml) | 1.15 (.92–1.44) |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio; ART, antiretroviral therapy; CAD, Canadian dollars; CI, confidence interval; ESLD, end-stage liver disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Smoking marijuana seemed to accelerate progression to a clinical diagnosis of cirrhosis (HR, 1.33 per 10 joints/week; 95% CI, 1.09–1.62). However, lagging the exposure attenuated this association (HR, 1.12; 95% CI, .94–1.34). Marijuana smoking was also associated with a slightly increased risk of progression to clinically diagnosed cirrhosis and ESLD combined: hazard ratio 1.13 (95% CI, 1.01–1.28). This association was no longer significant when marijuana exposure was lagged (HR, 1.10; 95% CI, .95–1.26). Baseline APRI score was a strong predictor of reaching an APRI score of 1.5 or 2, and of a clinical diagnosis of cirrhosis.
Dose-response Relationship With APRI Score
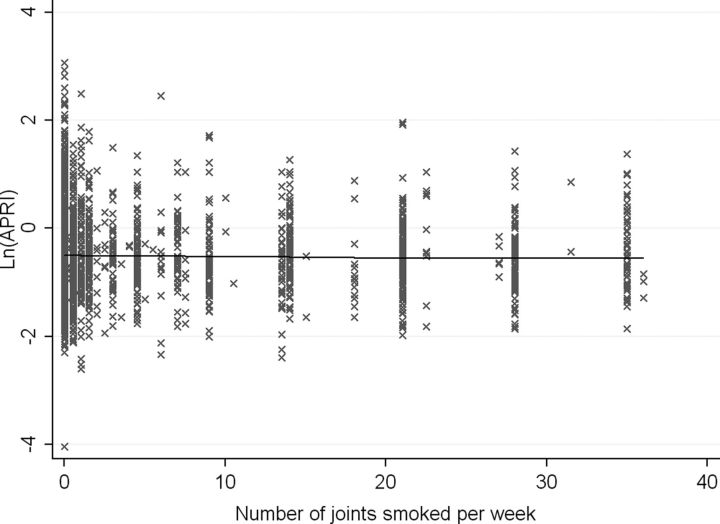
There was no evidence of a dose-response relationship between marijuana and APRI scores in follow-up. Figure 2 illustrates that the APRI score would not be expected to change significantly with increased smoking in any of the joints/week intervals evaluated. The linear spline regression model did not provide a better fit to the data over a simple linear regression.
Figure 2.
Relationship between the number of joints smoked per week and the aspartate aminotransferase-to-platelet ratio (APRI) score, linear spline regression model with knots at 7 and 21 joints/wk. There was no difference in the slope of ln(APRI) observed with increasing marijuana use. The ln(APRI) score would increase by 0.003 units (95% confidence interval [CI], −.001, .006) for each additional joint smoked per week for someone smoking between 0 and 7 joints/wk. For someone smoking between 7 and 21 joints/wk, it would increase by 0.004 units (95% CI, −.020, .028) and would decrease by .009 units (95% CI, −.024, .006) for someone smoking more than 21 joints/wk. Abbreviation: APRI, aspartate aminotransferase-to-platelet ratio.
DISCUSSION
In this first longitudinal study to our knowledge of marijuana smoking and risk of liver disease among HIV-HCV coinfected persons without significant fibrosis at baseline, we found no evidence that cannabis smoking increases the risk of progression to significant liver fibrosis or cirrhosis as measured by the standard APRI cutoffs. Furthermore, there was no evidence of any dose-response relationship with increasing cannabis use on APRI score. In addition, we did not observe any effect of marijuana use on the development of ESLD. We did observe a slight increase in the risk of progression to clinically diagnosed cirrhosis or cirrhosis and ESLD combined with high levels of marijuana smoking (33% or 13% increase for each additional 10 joints/week, respectively). However, this association disappeared after lagging the exposure, suggesting that previous cross-sectional studies reporting an association between marijuana smoking and liver fibrosis may be biased by reverse causation due to self-medication with marijuana for relief of symptoms related to significant liver fibrosis.
The 2 principal studies implicating marijuana as an independent risk factor for liver fibrosis were cross-sectional in design. Hézode et al [13] estimated fibrosis progression rates among 270 HCV monoinfected patients undergoing biopsy in a single center, which were correlated with history of marijuana use obtained contemporaneously with performance of the liver biopsy. They found daily cannabis use was associated with values of fibrosis progression rates >0.074 Metavir U/yr and with severe fibrosis (≥F3), OR, 3.4 (1.5–7.4) and 2.3 (1.1–4.8), respectively. Ishida et al [15] studied 204 consecutive HCV chronically infected patients recruited from community-based clinics who had undergone a liver biopsy; 21% were HIV coinfected. They found a strong association between daily cannabis use and moderate to severe fibrosis (OR, 6.78; 95% CI, 1.89–24.3) compared to mild fibrosis, but little association was apparent between cannabis use and the presence of mild fibrosis compared to no fibrosis. They concluded that cannabis may have little or no influence on the initiation of fibrosis, but once fibrosis is present, it may be an important cofactor in fibrosis progression.
To our knowledge, the only longitudinal study published described a small cohort of 58 HIV/AIDS patients recruited from an outpatient clinic and followed for 12 months and reported a non-statistically significant decrease in liver enzymes (ALT and AST) among dronabinol and/or marijuana users over the span of 1 year [16]. Finally, Hézode et al [14] estimated the association between cannabis use and the presence of marked steatosis (≥30% of hepatocytes containing cytoplasmic fat vacuoles) in a cross-sectional study of 315 HCV monoinfected patients undergoing biopsy. They found an OR of 0.5 (0.1–1.8) for occasional cannabis use and of 2.1 (1.01–4.5) for daily cannabis use compared no use.
Reported use for symptom relief was very prevalent suggesting that the association of daily cannabis use and more advanced fibrosis may, in fact, be related to an increased use for symptom management as disease advances. Interestingly, Ishida et al [15] found that daily cannabis users had lower body mass index (BMI) and were much more likely to have medically prescribed cannabis (57% vs 9%), suggesting they may have been experiencing more symptoms.
The cannabinoid system consists of 2 receptors (CB1 and CB2) to which cannabinoids can bind [11]. Depending on which receptor is expressed, cannabinoids could have opposite effects on the liver. Antifibrogenic and antiinflammatory effects of CB2 receptor have been observed in mice [10, 21–23]. Cannabinoids have been shown to decrease oxidative/nitrative stress and cell death [8], normalize liver enzymes in mice [9], and present antiinflammatory properties such as: (1) suppression of macrophage function, of antigen presentation and of chemokine production by human B cells; (2) inhibition of macrophage nitric oxide production, of cytotoxic T-cell activity and of T lymphocytes proliferative responses; and (3) regulation of tumor necrosis factor, interleukin-1, and interferon gamma production by human peripheral blood mononuclear cells [8, 24, 25]. However, expression of the CB1 receptor seems to have pro-fibrogenic properties [10, 26]. In cell culture, cannabidiol induces death of hepatic stellate cells, activation of which contributes to development of fibrosis [7]. The role of CB1/CB2 receptors expression and ratio is unclear in HCV progression. However, levels of CB1 are 6 times higher in chronic HCV patients than in controls, and twice higher in cirrhotic patients than in those at a low fibrosis stage [27–29]. In this study, we selected a population with evidence of chronic HCV infection, thus more likely to express high levels of profibrogenic CB1 receptors. It is also possible that we favored the inclusion of those with higher CB2 expression by selecting a population free of significant fibrosis and ESLD. However, this is unlikely to have biased our results because we were interested in studying progression to liver disease.
Our study has several strengths. It is a large, prospective, cohort study that is broadly representative of HIV-HCV coinfected persons in care in Canada. In previous studies, patients were only selected based on having undergone liver biopsy, which potentially introduces selection bias. Indeed, excluded patients in the Ishida study were significantly less likely to use marijuana. We assessed marijuana use and other potential confounders such as alcohol use and HIV disease stage concurrently at each study visit and exposures were updated longitudinally, thus limiting the potential for reverse causality. In addition to using a noninvasive surrogate for significant fibrosis and cirrhosis, we corroborated our results with clinical outcomes.
There are several limitations worth noting. We used APRI as a noninvasive surrogate for significant liver fibrosis, which may underestimate the degree of fibrosis present and may be influenced by factors other than fibrosis that affect AST and platelet values. The area under the receiver operating characteristic (AUROC) curve of APRI is 0.77 for significant fibrosis and 0.83 for cirrhosis without significant change in accuracy in HIV-HCV coinfected as compared to HCV monoinfected patients [30]. APRI is highly predictive of liver-related and all-cause mortality in HIV-HCV coinfection [19, 31, 32]. However, the reference standard for the diagnosis of significant hepatic fibrosis, liver biopsy, is invasive, costly and prone to sampling error and therefore not amenable to be used repeatedly [33]. Given the 18 years median duration of HCV infection, it is expected that many participants would have some degree of fibrosis at baseline. For this reason we also adjusted for baseline APRI in multivariate models, itself a strong predictor of liver fibrosis progression.
Although the relatively short follow-up time and the lack of information on duration of smoking before cohort entry do not allow us to make inference about long-term use of marijuana, the results of this study could still be valuable to clinicians who need to provide advice to their patients about immediate risks of marijuana smoking. Longer follow-up would be very valuable, however, to confirm our findings for extended exposure.
Clinical outcomes were relatively rare over the course of this study so it remains possible that we have missed a true effect (type II error) that may have been present if follow-up were extended so as to capture more events. However, the upper bounds of the 95% confidence intervals we observed are not consistent with an effect anywhere near as large as those previously reported (ORs of 3.4 and 6.78) –our study had 100% power to detect such a large effect for APRI ≥ 1.5 and APRI ≥ 2. Thus, if there is any effect of marijuana exposure it is likely to be quite small and only in more advanced disease. Indeed, our estimates are in line with lower bounds of the 95% confidence intervals reported from previous studies (ie, 1.01 and 1.89) and are much less than those for known important risk factors such as alcohol use.
We lacked detailed information on marijuana use history before cohort entry and therefore were only able to lag marijuana exposure by 6 months. However, in analyses when exposures were lagged by this amount, risks were attenuated. It is therefore unlikely that even more remote use would be expected to have had an effect. Moreover, exploratory analyses showed that current exposure provided the best fit to the data. We may have underestimated cannabis use as we did not collect information about ingested marijuana. We were also unable to investigate the effect of prescribed cannabinoid use such as marinol, nabilone, or sativex. However, use of these drugs was limited in our population.
It remains possible that the risk associated with cannabis exposure differs among HIV coinfected persons for whom there may be other more important predictors of liver disease. However, our analyses were controlled for antiretroviral use, and time updated CD4 cell count and HIV virologic control in addition to alcohol and drug use, which might be more common in our population. Finally, we could not assess the role of hepatic steatosis and insulin resistance, both important predictors of fibrosis progression [34, 35]. As marijuana use has been associated with the presence of steatosis, failure to account for steatosis would likely have biased our results away from the null rather than masking an effect of marijuana on fibrosis progression. Including BMI in the models did not change results (not shown).
To conclude, in this first prospective evaluation of liver disease progression among HIV-HCV infected persons, we could not demonstrate any important effect of marijuana on liver disease outcomes. A causal association is unlikely: hazard ratios were weak and most importantly were attenuated when accounting for temporality in the exposure-disease relationship and there was no dose-response relationship. It is likely that previous studies have been biased by reverse causality as patients use more marijuana to relieve symptoms as liver disease progresses.
Notes
Acknowledgments. The authors thank Valérie Martel Laferrière for her comments on the manuscript. The authors also thank Alex Schnubb, Manon Desmarais, Curtis Sikora, Christine O'Reilly, Brenda Beckthold, Heather Haldane, Laura Puri, Nancy McFarland, Claude Gagne, Elizabeth Knight, Lesley Gallagher, Warmond Chan, Sandra Gordan, Judy Latendre-Paquette, Natalie Jahnke, Viviane Josewski, Evelyn Mann, and Anja McNeil for their assistance with study coordination, participant recruitment and care.
The Canadian Coinfection cohort investigators (CTN222) are Jeff Cohen, Windsor Regional Hospital Metroplitan Campus, Windsor, Ontario, Canada; Brian Conway, Downtown IDC, Vancouver, British Columbia, Canada; C. C., Ottawa General Hospital, Ottawa, Ontario, Canada; Pierre Côté, Clinique du Quartier Latin, Montreal, Quebec, Canada; J. C., Montreal General Hospital, Montreal, Quebec, Canada; John Gill, Southern Alberta HIV Clinic, Calgary, Alberta, Canada; Mark Tyndall, Native Health Centre, Vancouver, British Columbia, Canada; Shariq Haider, McMaster University, Hamilton, Ontario, Canada; Marianne Harris, St Paul's Hospital, Vancouver, British Columbia, Canada; David Hasse, Capital District Health Authority, Halifax, Nova Scotia, Canada; Julio Montaner, St Paul's Hospital, Vancouver, British Columbia, Canada; E. E. M. M., McGill University, Montreal, Quebec, Canada; N. P., Oak Tree Clinic, Vancouver, British Columbia, Canada; Annita Rachlis, Sunnybrook and Women's College Health Sciences Centre, Toronto, Ontario, Canada; Roger Sandre, HAVEN Program, Sudbury, Ontario, Canada; Danielle Rouleau, Centre Hospitalier de l'Université de Montréal, Montreal, Quebec, Canada; David Wong, University Health Network, Toronto, Ontario, Canada; M.W.H., British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; and S. W., Toronto General Hospital, Toronto, Ontario, Canada.
Financial support. This work was supported through grant support from the Fonds de recherche du Québec en santé, Réseau SIDA/maladies infectieuses (FRSQ); the Canadian Institutes of Health Research (CIHR MOP-79529); the Canadian Institutes of Health Research Canadian HIV Trials Network (CTN222) and salary awards from the FRQS (Doctoral Training Award to L. B. and Chercheur-boursiers cliniciens senior career award to M. B. K.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004;18:2039–45. doi: 10.1097/00002030-200410210-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Adlaf E, Begin P, Sawka E. Canadian Addiction Survey (CAS): A national survey of Canadians’ use of alcohol and other drugs: Prevalence of use and related harms: Detailed report. Ottawa: Canadian Centre on Substance Abuse; 2005. [Google Scholar]
- 6.Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDS. 2004;18:215–28. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- 7.Lim MP, Devi LA, Rozenfeld R. Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2011;2:e170. doi: 10.1038/cddis.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhopadhyay P, Rajesh M, Horvath B, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–81. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avraham Y, Grigoriadis N, Poutahidis T, et al. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162:1650–8. doi: 10.1111/j.1476-5381.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parfieniuk A, Flisiak R. Role of cannabinoids in chronic liver diseases. World J Gastroenterol. 2008;14:6109–14. doi: 10.3748/wjg.14.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol. 2011;163:1432–40. doi: 10.1111/j.1476-5381.2011.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L, Quinn MA, Frampton GA, Golden LE, DeMorrow S. Recent advances in the understanding of the role of the endocannabinoid system in liver diseases. Dig Liver Dis. 2011;43:188–93. doi: 10.1016/j.dld.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hezode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 14.Hezode C, Zafrani ES, Roudot-Thoraval F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008;134:432–9. doi: 10.1053/j.gastro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Ishida JH, Peters MG, Jin C, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69–75. doi: 10.1016/j.cgh.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitfield RM, Bechtel LM, Starich GH. The impact of ethanol and Marinol/marijuana usage on HIV+/AIDS patients undergoing azidothymidine, azidothymidine/dideoxycytidine, or dideoxyinosine therapy. Alcohol Clin Exp Res. 1997;21:122–7. [PubMed] [Google Scholar]
- 17.Klein MB, Saeed S, Yang H, et al. Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol. 2010;39:1162–9. doi: 10.1093/ije/dyp297. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Bacchetti P, Tien PC, Seaberg EC, et al. Estimating past hepatitis C infection risk from reported risk factor histories: implications for imputing age of infection and modeling fibrosis progression. BMC Infect Dis. 2007;7:145. doi: 10.1186/1471-2334-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics Canada. Ottawa: 2012. Low Income Lines, 2010 to 2011. Income Research Paper Series. [Google Scholar]
- 21.Avraham Y, Amer J, Doron S, et al. The direct pro-fibrotic and indirect immune anti-fibrotic balance of blocking the cannabinoid CB2 receptor. Am J Physiol Gastrointes Liver Physiol. 2012;302:G1364–72. doi: 10.1152/ajpgi.00191.2011. [DOI] [PubMed] [Google Scholar]
- 22.Munoz-Luque J, Ros J, Fernandez-Varo G, et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008;324:475–83. doi: 10.1124/jpet.107.131896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang HC, Wang SS, Hsin IF, et al. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248–58. doi: 10.1002/hep.25625. [DOI] [PubMed] [Google Scholar]
- 24.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42(11 Suppl):11S–9S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 25.Croxford JL, Yamamura T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Giannone FA, Baldassarre M, Domenicali M, et al. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest: J Techl Methods Pathol. 2012;92:384–95. doi: 10.1038/labinvest.2011.191. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda M, Kitaoka A, Machida K, et al. Association between lipid accumulation and the cannabinoid system in Huh7 cells expressing HCV genes. Int J Molecular Med. 2011;27:619–24. doi: 10.3892/ijmm.2011.622. [DOI] [PubMed] [Google Scholar]
- 28.van der Poorten D, Shahidi M, Tay E, et al. Hepatitis C virus induces the cannabinoid receptor 1. PLoS One. 2010;5:e12841. doi: 10.1371/journal.pone.0012841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Liu Y, Huang S, et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171:31–8. doi: 10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44:463–9. doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 32.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–53. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 33.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–42. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Hull MW, Rollet K, Moodie EE, et al. Insulin resistance is associated with progression to hepatic fibrosis in a cohort of HIV/hepatitis C virus-coinfected patients. AIDS. 2012;26:1789–94. doi: 10.1097/QAD.0b013e32835612ce. [DOI] [PubMed] [Google Scholar]