Abstract
This study sought to investigate the clinical efficacy and safety of combined oral therapy with sildenafil and doxazosin GITS compared to sildenafil monotherapy in treating Chinese patients with erectile dysfunction (ED) and lower urinary tract symptoms secondary to benign prostatic hyperplasia (BPH/LUTS). The trial was conducted in hospitals in Beijing, Shanghai, Changsha, Wuhan and Guangzhou, five major cities in China. A total of 250 patients diagnosed with ED and BPH/LUTS aged 50–75 years, and who had International Index of Erection Function-5 (IIEF-5) scores ≤21 and International Prostate Symptom Score (IPSS) ≥10 points, were enrolled and randomly divided into Group A (168 cases; doxazosin GITS 4 mg once daily plus sildenafil 25–100 mg on demand) and Group B (82 cases; sildenafil 25–100 mg on demand). Efficacies were evaluated by IIEF-5 and IPSS scores and a quality of life (QoL) questionnaire, and adverse effects were evaluated during the treatment period. There were no statistically significant differences in mean age, and IIEF-5, IPSS and QoL scores pre-treatment between the two groups. After treatment, IIEF-5, IPSS and QoL scores were significantly improved in Group A, while only IIEF-5 scores were significantly improved in Group B compared with pre-treatment. There were no significant differences in side effects between the two groups. The results indicated that combined therapy with sildenafil and doxazosin GITS for the treatment of ED and BPH/LUTS is safe and effective compared to sildenafil monotherapy.
Keywords: doxazosin GITS, erectile dysfunction, IPSS, lower urinary tract symptoms, quality of life, sildenafil
Introduction
With increasing human life expectancies, age-related diseases have become an important clinical issue. The medical profession will be faced with the task of ensuring that the ageing population remains healthy and maintains a good quality of life (QoL). From the results of many epidemiological studies, we know that erectile dysfunction (ED), benign prostatic hyperplasia (BPH), cardiovascular disease and depression are the four major non-cancerous diseases in males over 50 years old, and the morbidity of those diseases increases with advancing age.1 The rates of ED and BPH in men over 40 years of age are 52% and 56%, respectively. Approximately half of men over 60 years old suffer from BPH, and 15%–30% of these men have lower urinary tract symptoms (LUTS).2, 3 Studies have indicated that LUT severity may be related to ED prevalence.4 These studies have indicated that ED and BPH coexist in ageing males at a high rate, and that the combination of these conditions seriously interferes with daily life, decreasing the QoL of ageing men. BPH can have a profound effect on a patient's QoL and sexual function. However, little clinical research has focused on ED combined with BPH /LUTS.5
Although the mechanism of ED and BPH/LUTS remain to be investigated, some of studies have been reported that smooth muscle dysfunction in prostrate and/or penis may induce dysuria and/or ED due to increased Rho-kinase channel activity, which may be related to further constriction of the smooth muscle and decrease in nitric oxide synthese activity and utility affects NO–cyclic guanosine monophosphate signalling channels, altering endothelial cell function and leading to arteriosclerosis, autonomic nervous system overactivity and disturbance,6 and endothelial nitric oxide synthase 894T allele gene susceptive may have relationship to ED and BPH/LUTS.7
We hypothesize that combined oral therapy with sildenafil and doxazosin will be more effective than sildenafil alone for improving erectile function and LUTS suggestive of BPH, thus improving QoL in patients with ED and BPH/LUTS.
Materials and methods
Patient enrolment
The trial was conducted in five centres of medical university-associated hospitals located in Beijing (northern China), Shanghai (eastern China), Changsha (southern China), Wuhan (central China) and Guangzhou (southeastern China) from September 2007 to October 2009. Approvals were obtained from local ethics committees of each hospital. All patients gave written informed consent prior to the study. A total of 250 patients aged 50–75 years who were diagnosed ED and BPH/LUTS were enrolled in this study and 203 patients (81.2%) completed the entire follow-up evaluation.
Patients were recruited for the trial according to their International Index of Erection Function-5 scores (IIEF-5≤21) and International Prostate Symptom Scores (IPSS≥10 points). Patients who had no medical, surgical or experimental interventions for BPH previous 2 weeks to the study were eligible to participate. Other inclusion criteria included a maximum urinary flow rate (Qmax) ≤15 ml s−1 for a voided volume ≥150 ml with a post-voiding volume <150 ml, a prostate volume ≥25 ml and a serum prostate-specific antigen level <4 ng ml−1. All patients were heterosexual with stable partners. A normal blood testosterone level was also used as an inclusion criterion.
Exclusion criteria included patients with significant cardiovascular disease, uncontrolled diabetes mellitus, neurological and psychiatric disorders, previous genitourinary surgery, and history of hypersensitivity and contraindication to one of the study drugs or participation in another clinical trial in 3 months prior to the study.
Study design
This study was a two-armed, randomized, parallel, non-inferior, comparative study of doxazosin (Cardura XL; Pfizer Inc., Dalian, China) 4 mg once daily plus sildenafil (Viagra; Pfizer Inc.) 25–100 mg on demand compared with sildenafil monotherapy in the treatment of patients with ED and BPH/LUTS. No placebo arm was included because of the difficulty of placebo preparation.
Patients who fulfilled the entry criteria at selection were randomized to combined-therapy group (Group A, n=168) or monotherapy group (Group B, n=82). In Group A, patients received doxazosin GITS (4 mg d−1) plus sildenafil (25–100 mg on demand) with at least 6-h interval between the administrations of the two drugs, while in Group B, patients only received sildenafil treatment (25–100 mg on demand). According to the IIEF-5 score, each group was divided into subgroups for mild (17–21), moderate (12–16) and severe (1–11) ED. According to the IPSS score, each group was divided into subgroups for moderate (10–19) and severe (≥20).
Serum prostate-specific antigen, testosterone and uroflowmetry testing were also conducted during the screening period. Clinical efficacies assessments for ED and BPH/LUTS were evaluated by IIEF-5, IPSS and QoL questionnaires, and follow-up assessments were performed by telephone inquiries and email communication. Safety profiles were assessed by physical examination (heart rate and blood pressure), laboratory tests and monitoring clinical adverse events.
Assessment
Clinical efficacy assessments for ED and BPH/LUTS were evaluated by IIEF-5, IPSS and QoL. The results of IIEF-5, IPSS and QoL were used to evaluate related symptoms at pre-treatment (Pre Tx), 3 and 6 months post-treatment in both groups. The IPSS was used to assess LUTS and is the internationally accepted standard questionnaire for assessing BPH/LUTS. It is a validated, seven-item scale that assesses the severity of incomplete emptying, urinary frequency, intermittency, urgency, weak stream and nocturia. The seven items have an ordered categorical response that can be scored from 0 to 5, with an overall score of 0–35. The severity of symptoms was classified as moderate (10–19) or severe (≥20).
Sexual function was assessed using the IIEF-5. The IIEF-5 questionnaire (also referred to as the Sexual Health Inventory of Men) is an abridged, five-item version of the IIEF-15 questionnaire. It is a validated, multidimensional questionnaire that is a sensitive indicator of changes in erectile function and treatment outcomes. It is scored from 1 to 5. The total IIEF-5 score, calculated by totalling the responses to all five questions, is interpreted as follows: normal (22–25); mild ED (17–21); moderate ED (12–16); and severe ED (1–11). Safety is assessed as the incidence of adverse events.8
QoL was assessed using QoL scores to evaluate the degree of negative impact by BPH/LUTS.
Statistical analysis
Data are expressed as mean±s.d. A statistical difference analysis was carried out by a chi-square test, analysis of covariance and independent and paired t-test. All analyses were two-tailed, with a significance level of 5%. Analyses were performed using the SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Among the 250 cases enrolled, 221 (88.4%) completed the 3-month follow-up evaluation (Group A: 149/168; Group B: 72/82), and 203 patients (81.2%) finished the 6-month follow-up evaluation (Group A: 137/168; Group B: 66/82). The average patient age and body mass index for each group and the subgroup ratio of ED and BPH/LUTS severity between the two groups were not significantly different (P=0.315, P=0.441, P=0.535 and P=0.431, respectively). The Qmax and post-void residual (PVR) also showed no significant differences between the two groups (P=0.074 and P=0.181, respectively) (Table 1).
Table 1. Patients' characteristics (total 250 who were enrolled).
| Group A (n=168) | Group B (n=82) | P value | |
|---|---|---|---|
| Hospital location | 55 (32.7) | 24 (29.3) | |
| Beijing, n (%) | 26 (15.5) | 12 (14.6) | |
| Shanghai, (n (%) | 20 (11.9) | 10 (12.2) | |
| Changsha, n (%) | 35 (20.8) | 16 (19.5) | |
| Wuhan, n (%) | 32 (19.1) | 20 (24.4) | |
| Guangzhou, n (%) | |||
| Age (years) | 61.7±5.3 | 60.8±5.9 | 0.315 |
| Body mass index (kg m−2) | 23.5±3.9 | 24.0±3.5 | 0.441 |
| Qmax (ml s−1) | 10.7±2.1 | 11.2±1.3 | 0.074 |
| PVR (ml) | 23.0±14.1 | 20.5±11.3 | 0.181 |
| BPH/LUTS severity | |||
| Moderate (IPSS 10–19), n (%) | 102 (60.8) | 54 (65.8) | 0.431 |
| Severe (IPSS ≥20), n (%) | 66 (39.2) | 28 (34.2) | |
| ED severity | |||
| Mild (IIEF-5 17-21), n (%) | 13 (7.7) | 8 (9.7) | 0.535 |
| Moderate (IIEF-5 12–16), n (%) | 126 (75.0) | 56 (68.3) | |
| Severe (IIEF-5 5-11), n (%) | 29 (17.3) | 18 (22.0) |
Abbreviations: BPH/LUTS, lower urinary tract symptoms secondary to benign prostatic hyperplasia; ED, erectile dysfunction; IIEF-5, International Index of Erection Function-5; IPSS, International Prostate Symptom Score; PVR, post-void residual; Qmax, a maximum urinary flow rate.
Group A, sildenafil and doxazosin GITS combined therapy. Group B, sildenafil monotherapy. Values are expressed as mean±s.d. unless stated otherwise. Independent and paired t-test was carried out for the agents of age, body mass index, Qmax and PVR. Chi-square test was carried out for the agent of ED and BPH/LUTS severity.
IIEF-5 total score changes
In Group A, the Pre Tx, 3-month, 6-month scores post-treatment were 13.0±3.0, 18.4±4.4 and 19.9±4.4, and in Group B, they were 12.4±3.6, 16.8±5.6 and 17.2±5.7, respectively. IIEF-5 total scores evaluated at Pre Tx showed no significant difference between the two groups (P=0.202). IIEF-5 total scores were significant increases in the 3- and 6-month post-treatment compared to the Pre Tx (all P=0.000) in both groups. Score changed in time-dependent manner (P=0.0001). IIEF-5 scores, as evaluated at 3 and 6 months post-medication in Group A, were markedly improved compared to those in Group B (P=0.022 and P=0.0001, respectively) (Figure 1a).
Figure 1.
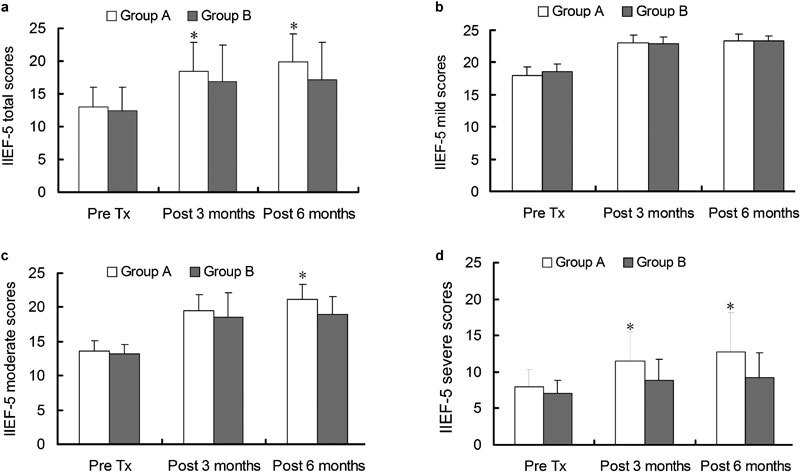
(a) IIEF-5 total score changes over time. Significant improvements with sildenafil and doxazosin GITS combined therapy (Group A) versus sildenafil monotherapy (Group B) were observed both at 3 and 6 months post-medication. (b) IIEF-5 mild score comparison between the two groups. In mild ED patients (IIEF-5: 17–21), no significant improvements with combined therapy versus monotherapy were observed both at 3 and 6 months post-medication (P>0.05). (c) IIEF-5 moderate score comparison between the two groups. In moderate ED patients (IIEF-5: 12–16), significant improvements with combined therapy versus monotherapy were observed at 6 months (P=0.000) post-medication but not at 3 months (P>0.05). (d) Severe ED patients IIEF-5 score comparison between the two groups. In severe ED patients (IIEF-5: 5–11), significant improvements with combined therapy versus monotherapy were observed both at 3 months (P=0.008) and 6 months (P=0.001) post-medication. *P<0.05, compared with Group B. Statistical difference analysis was carried out by analysis of variance of repeated data measured. ED, erectile dysfunction; IIEF-5, International Index of Erection Function-5; Pre Tx, pre-treatment.
IIEF-5 score changes in subgroups
In both Group A and Group B, all mild, moderate and severe ED patients showed significant improvements in IIEF-5 scores at 3- and 6-month post-medication compared to pre-medication (all P=0.000 in Group A and all P<0.05 in Group B). In IIEF-5 scores between two groups at the end of 3-month medication, there was a significant difference for severe ED patients (P=0.008) but not for mild or moderate ED patients (P=0.875 and P=0.081, respectively). At the end of 6-month medication, there was a significant difference for severe and moderate ED patients (P=0.001 and P=0.000, respectively) but not for mild ED patients (P=0.959) (Figure 1b–d).
IPSS total score changes
In Group A, the pre-medication, 3- and 6-month post-medication IPSS scores were 18.4±5.8, 13.6±5.8 and 10.2±5.4, and in Group B, they were 16.9±4.5, 16.8±5.3 and 17.2±5.8, respectively. The Pre Tx IPSS total scores did not differ significantly between the two treatment groups (P=0.080). In Group A, the 3-month post-treatment score was significantly reduced compared to the pre-medication (P<0.05), and the 6-month score post-treatment was also reduced compared to the 3-month score (P<0.05). Compared to the pre-medication score, there were no marked changes in the 3- and 6-month post-treatment scores (P=0.385 and P=0.233, respectively) in Group B. Significant improvements in Group A versus Group B were also observed at both 3 and 6 months post-treatment (P<0.05). (Figure 2a).
Figure 2.
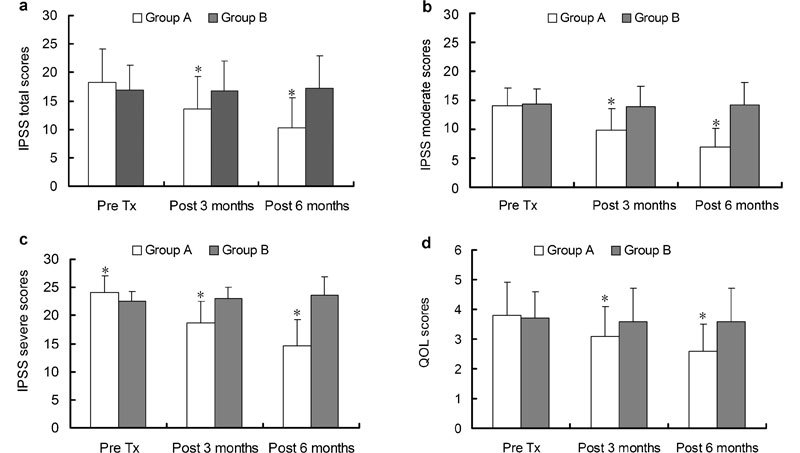
(a) IPSS total score changes over time. Significant improvements with sildenafil and doxazosin GITS combined therapy (Group A) versus sildenafil monotherapy (Group B) were observed both at 3 and 6 months post-medication. (b) Moderate BPH/LUTS patients' IPSS score comparison between the two groups. In moderate BPH/LUTS patients (IPSS: 10–19), significant improvements with Group A versus Group B were observed both at 3 and 6 months post-medication. (c) Severe BPH/LUTS patients' IPSS score comparison between the two groups. In severe BPH/LUTS patients (IPSS ≥20), significant improvements with Group A versus Group B were observed at pre-medication, 3 and 6 months post-medication. (d) QoL total score changes over time. Significant improvements with Group A versus Group B were observed both at 3 and 6 months post-medication. *P<0.05, compared with Group B. BPH/LUTS, lower urinary tract symptoms secondary to benign prostatic hyperplasia; IPSS, International Prostate Symptom Score; Pre Tx, pre-treatment; QoL, quality of life.
IPSS score changes in subgroups
When the IPSS total score was separated into moderate (10–19) and severe (≥20) subgroups, in Group A, there was a significant reduction among the Pre Tx, 3- and 6-month post-treatment scores for moderate and severe patients (all P=0.000). In Group B, there were no significant differences among the Pre Tx, 3- and 6-month post-treatment scores (all P>0.05) for moderate patients, and in severe patients, both the 3- and 6-month post-treatment scores increased, and at 3-month post-treatment evaluation, there was no significant difference (P=0.076), but after 6 months post-treatment, the symptoms were markedly worse (P=0.013). In moderate patients (IPSS 10–19), significant improvements with Group A versus Group B were observed at both 3 and 6 months post-treatment (Figure 2b). In severe patients (IPSS ≥20), significant improvements with Group A versus Group B were observed at Pre Tx, 3 and 6 months post-treatment (Figure 2c).
QoL score changes
QoL total scores Pre Tx showed no significantly difference between the two treatment groups. In Group A, the 3- and 6-month post-treatment scores were significantly reduced compared to Pre Tx score (both P=0.000). In Group B, there was no significant difference in the 3- and 6-month post-treatment scores compared to Pre Tx (both P>0.05). Patients' life quality was improved with QoL total scores being significantly decreased in Group A compared to that in Group B at both 3 and 6 months post-treatment (Figure 2d).
Sexual intercourse changes
At the end of 6-month therapy, the percentage of patients who performed in sexual intercourse more than four times per month was 64.2% (Group A) and 60.6% (Group B); there was no significant difference between the two groups (P=0.616), but the percentage for each group was significantly increased compared to the Pre Tx (Group A, 30.1% Group B, 28.9% both P=0.000).
Increases in the frequency of penetration (IIEF-5, Q3) and maintained erections (IIEF-5, Q4) were greater in Group A (Q3+Q4≥8, 60.2%) than in Group B (Q3+Q4≥8, 42.3%) (P=0.011).
Safety
The number of patients who complained about adverse effects at the end of 6 months of therapy were 26 (15.4%) in Group A and 12 (14.6%) for Group B (P>0.05), and some patients complained two or more kinds of adverse effects either in Group A or in Group B (Table 2). However, all symptoms were mild, and no participant left the study owing to adverse symptoms. The average heart rate and systolic and diastolic pressures at Pre Tx were 74.7±5.3 bpm, 123.8±9.0 mmHg and 71.2±6.8 mmHg, respectively, in Group A, and 74.6±5.2 bpm, 118.0 ±11.5 mmHg and 70.4±7.1 mmHg, respectively, in Group B. At the end of 3-month mediation, the values of Group A were 75.2±4.8 bpm, 124.0±9.1 mmHg and 71.1±6.7 mmHg (compared to Pre Tx, P=0.353, 0.057 and 0.863, respectively) and in Group B, the values were 74.0± 5.1 bpm, 117.8±11.3 mmHg and 70.2±7.3 mmHg (compared to Pre Tx, P=0.434, 0.103 and 0.787, respectively). At the end of 6-month treatment, the values in Group A were 75.7±4.9 bpm, 123.9±9.1 mmHg and 70.9±6.6 mmHg (compared to Pre Tx, P=0.087, 0.198 and 0.390, respectively) and in group B, they were 75.0±5.8 bpm, 119.2±11.7 mmHg and 69.9±7.0 mmHg, respectively (compared to Pre Tx, P=0.725, 0.083 and 0.349, respectively), and there was no statistical significance. Mean laboratory parameters fell within normal ranges at all times, and changes in these parameters during the study period were minor and not considered clinically relevant.
Table 2. Treatment-emergent adverse events during 6-months medication.
| Adverse event | Group A (n=168) | Group B (n=82) |
|---|---|---|
| Headache, n (%) | 9 (5.4) | 5 (6.1) |
| Dizzy, n (%) | 8 (4.8) | 4 (4.9) |
| Facial flushing, n (%) | 6 (3.6) | 4 (4.9) |
| Palpitation, n (%) | 6 (3.6) | 2 (2.4) |
| Dyspepsia, n (%) | 5 (3.0) | 2 (2.4) |
| Diarrhea, n (%) | 4 (2.4) | 1 (1.2) |
| Acratia, n (%) | 2 (1.2) | 1 (1.2) |
| Abdominal pain, n (%) | 1 (0.6) | 0 (0) |
Group A, sildenafil and doxazosin GITS combined therapy. Group B, sildenafil monotherapy. Values (n) shown are the numbers of patients affected (%).
Discussion
BPH and ED are common diseases in ageing males. LUTS are common in patients with BPH, and the probability of BPH occurring concurrently with ED is high. International epidemiological studies have shown that there is a strong relationship between LUTS and erectile dysfunction, and it is not influenced by age or comorbidities.9, 10 Phosphodiesterase type 5 (PDE5) inhibitors (e.g., sildenafil) are first-line medications for ED therapy, and α1-adrenergic receptor inhibitors (e.g., doxazosin) have been shown to be effective in treating BPH/LUTS; close observation of pathogenically conditions and medication-based treatments have been the first-line therapy for BPH/LUTS.11
A basic research study found that PDE4 and PDE5 are located in the prostate gland.12 The results of this study gave rise to the speculation that drugs such as PDE4 and PDE5 inhibitors, which elevate the intracellular levels of cyclic adenosine monophosphate and cyclic guanosine monophosphate, may influence smooth muscle tone; therefore, doxazosin reduces adrenergic tension and may increase the level of cyclic adenosine monophosphate in smooth muscle of corpus cavernosum to facilitate erectile function.
Kaplan et al.13 reported that after treatment with alfuzosin (10 mg once daily), sildenafil (25 mg once daily) or a combination of the two drugs for 12 weeks in patients with previously untreated LUTS and ED, improvements in IPSS were significant for all three treatments, but were the greatest for the combined therapy. Frequency, nocturia, PVR and Qmax were significantly improved with alfuzosin alone and with the combined treatment. Improvements in IIEF were significant for sildenafil alone, and greater with the combined treatment, but there was no significant for alfuzosin alone. Likewise, increases in the frequency of penetration and maintained erections were greater in the combination therapy than alfuzosin or sildenafil alone.13 These investigations demonstrated that the combined use of a PDE5 inhibitor and an α1-adrenergic receptor inhibitor might be more effective than monotherapy with either agent.
As effective medications for ED and BPH therapy, the combination therapy of doxazosin and sildenafil for refractory ED showed greater effectiveness than sildenafil alone. This finding correlates with the anti-adrenergic function of doxazosin, which decreases the tension of the sympathetic nervous system and facilitates the effect of sildenafil stimulating the non-adrenergic non-cholinergic nervous system, augmenting penile erectile function. Oger et al.14 reported that in both cavernosal and prostatic tissues, the combination of sildenafil and doxazosin exerted a greater relaxant effect on concentration–response curves of phenylephrine or norepinephrine (NE) compared to either compound alone. Sildenafil significantly enhanced the relaxant effect of doxazosin on electrical field stimulation-induced contractions in prostatic strips. Doxazosin significantly increased the ability of sildenafil to inhibit NE-induced contractions in cavernosal strips. And in vitro, a PDE5 inhibitor (tadalafil) significantly inhibited NE-induced prostatic strip contractions by reducing the maximal effect, whereas α1-blockers (alfuzosin) shifted concentration–response curves of NE to the right, and the combination of both drugs resulted in a greater relaxant effect.15
In our study, the average age, body mass index and subgroup ratios of patients in both groups showed no significant differences; therefore, we can eliminate the effects of age, medication absorption, distribution and disease severity on the observed efficacy. In addition, there was little difference in the total Pre Tx IPSS, QoL, IIEF-5, Qmax and PVR scores, indicating that the Pre Tx severity of ED, LUTS secondary to BPH and urination effects on QoL was similar for the two groups.
In our study, sildenafil shows marked efficacy on ED; when combined with doxazosin, it had more significantly improved IIEF-5 score. With increasing treatment time, both groups showed better improvements in IIEF-5 score, which may be associated with increases in the frequency of sexual intercourse. At the end of 6 months of therapy, the percentage of patients who had intercourse more than four times a month was 64.2% (combined-therapy group) and 60.6% (monotherapy group). Both percentages were significantly increased compared to Pre Tx, but in the combined-therapy group, the intercourse quality (Q3+Q4) improved to a greater extent than in the monotherapy group. Along with the increase in the frequency of sexual intercourse, the frequency of drug administration also increased. The 3- and 6-month post-medication IIEF-5 scores of the combined-therapy group were significantly improved compared to the scores of the monotherapy group, indicating that doxazosin may augment the effect of sildenafil. In patients with severe symptoms, this effect was more significant, and from the results of patients with severe, moderate and mild ED symptoms, we calculated that combined therapy is suitable for patients with severe and moderate symptoms; for patients with mild symptoms, either treatment is acceptable, depending on the severity of BPH. According to previously reported clinical evidence,16 for patients who failed to respond to monotherapy, a combined therapy can be administered.
It has been reported that regular treatment with PDE5 inhibitors is effective in treating LUTS secondary to BPH.17, 18 Uckert et al.19 reported that PDE5 inhibitors, by interfering with cyclic nucleotide-mediated pathways to elevate cyclic adenosine monophosphate and cyclic guanosine monophosphate, can reverse the tension induced by NE in isolated prostatic tissue.
Both moderate and severe patients receiving combined therapy showed significant improvements in LUTS secondary to BPH, and QoL was correlated with IPSS. For patients in the sildenafil group, after 3 and 6 months of therapy, the incidence of LUTS was not improved for moderate or severe patients, indicating that sildenafil administration on demand was not effective in treating LUTS. Particularly for patients with severe symptoms, as time passed, their symptoms were aggravated.
Sildenafil and doxazosin have been used in clinical therapies for many years. A number of patients have received each drug, and their adverse effects have been investigated comprehensively. The most commonly reported treatment-related sildenafil adverse events are headache (9%–39%), facial flushing (7%–33%), dyspepsia, dizziness, nasal congestion, abnormal vision and palpitation. Adverse events are related to the dose administered, mild in nature and self-healing by continuous use.20 Adverse effects of doxazosin during BPH therapy include dizziness, headache (10%), acratia, oedema (2.7%), dyspnea (2.6%), abdominal pain (2.4%), diarrhoea (2.3%); dizziness and dyspnea are related to the dose administered. These effects are usually mild to moderate.21 In our study, the incidence of adverse effects was lower than reported rates, all patients showed good drug tolerance, all adverse effects were mild, and no patients left the study because of adverse effects. These results might be due to the small number of participants, the relatively strict entry criteria and the good basic health conditions of the patients. It should also be noted that at least 6 hours passed between administrations of the two drugs. From the drugs' pharmacology, we know that either of them might affect haemodynamics, especially doxazosin, with respect to blood pressure. It has been reported that tadalafil (20 mg) could augment the hypotensive effects of doxazosin (8 mg).22 However, after the 3- and 6-month treatments, we found that there was no major elevation of participants' blood pressure or heart rate, which may be related to the relatively low dosage of doxazosin (4 mg) and the shorter half-life of sildenafil compared to tadalafil.
In our study, the lack of a placebo arm ultimately limits the possible evaluation of the efficacy of the therapy under study and leads to the inability to place this trial into context with other trials. In addition, the lack of a doxazosin monotherapy arm may prevent the evaluation of the true efficacy of combined therapy on BPH.
Conclusion
This study indicates that the combined therapy of sildenafil and doxazosin is an effective therapeutic schedule for treating ED with LUTS secondary to BPH, the improvement in ED symptoms is superior to that of sildenafil monotherapy, and the combined therapy is relatively safe.
Author contributions
ZCZ, JHL, JL, YXT, XZS, WDS and BG were mainly in charge of patients collection and follow-up. ZJ conducted statistic analysis of data and manuscript writing, and YLG and ZCX were instructors.
Acknowledgments
This study was supported by the Investigator Initiate Research Funding from Pfizer Inc. (Cardura XL; Pfizer Inc., Dalian, China).
Pfizer Inc. provided funding support for this study.
References
- Zakaria L, Anastasiadis AG, Shabsigh R. Common conditions of the aging male: erectile dysfunction, benign prostatic hyperplasia, cardiovascular disease and depression. Int Urol Nephrol. 2001;33:283–92. doi: 10.1023/a:1015292603884. [DOI] [PubMed] [Google Scholar]
- Oishi K, Boyle P, Barry MJ.Epidemiology and natural history of benign prostatic hyperplasia. Proceedings of the Fourth International Consultation on BPH; 2–5 July 1997; Paris, France Plymouth, UK; Health Publications; 1998, pp23–59. [Google Scholar]
- Glina S, Santana AW, Azank F, Mello LF, Moreira ED., Jr Lower urinary tract symptoms and erectile dysfunction are highly prevalent in ageing men. BJU Int. 2006;97:763–5. doi: 10.1111/j.1464-410X.2005.06008.x. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Wei JT, Althof SE, Seftel AD, Miner M, et al. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology. 2009;73:562–6. doi: 10.1016/j.urology.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Taylor JM, DeSouza R, Wang R. Common approach to managing lower urinary tract symptoms and erectile dysfunction. Asian J Androl. 2008;10:45–53. doi: 10.1111/j.1745-7262.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- Lee YC, Wu WJ, Liu CC, Wang CJ, Li WM, et al. The associations among eNOS G894T gene polymorphism, erectile dysfunction, and benign prostate hyperplasia-related lower urinary tract symptoms. J Sex Med. 2009;6:3158–65. doi: 10.1111/j.1743-6109.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Li MK, Garcia L, Patron N, Moh LC, Sundram M, et al. An Asian multinational prospective observational registry of patients with benign prostatic hyperplasia, with a focus on comorbidities, lower urinary tract symptoms and sexual function. BJU Int. 2008;101:197–202. doi: 10.1111/j.1464-410X.2007.07320.x. [DOI] [PubMed] [Google Scholar]
- Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, et al. Lower urinary tract symptoms and male sexual dysfunction: the Multinational Survey of the Aging Male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J Am Geriatr Soc. 2001;49:436–42. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia; 2003. Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- Uckert S, Küthe A, Jonas U. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001;166:2484–90. [PubMed] [Google Scholar]
- Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2008;53:1320. doi: 10.1016/j.eururo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Lecoz O, Lebret T, et al. Combination of doxazosin and sildenafil exerts an additive relaxing effect compared with each compound alone on human cavernosal and prostatic tissue. J Sex Med. 2009;6:836–47. doi: 10.1111/j.1743-6109.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Lebret T, Denoux Y, et al. Combination of alfuzosin and tadalafil exerts an additive relaxant effect on human detrusor and prostatic tissues in vitro. . Eur Urol. 2010;57:699–707. doi: 10.1016/j.eururo.2009.04.039. [DOI] [PubMed] [Google Scholar]
- de Rose AF, Giglio M, Traverso P, Lantieri P. Combined oral therapy with sildenafil and doxazosin for the treatment of non-organic erectile dysfunction refractory to sildenafil monotherapy. Int J Impot Res. 2002;14:50–3. doi: 10.1038/sj.ijir.3900815. [DOI] [PubMed] [Google Scholar]
- Broderick GA, Brock GB, Roehrborn CG, Watts SD, Elion-Mboussa A, et al. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunction. Urology. 2010;75:1452–8. doi: 10.1016/j.urology.2009.09.093. [DOI] [PubMed] [Google Scholar]
- Porst H, McVary KT, Montorsi F, Sutherland P, Elion-Mboussa A, et al. Effects of once-daily tadalafil on erectile function in men with erectile dysfunction and signs and symptoms of benign prostatic hyperplasia. Eur Urol. 2009;56:727–35. doi: 10.1016/j.eururo.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Uckert S, Sormes M, Kedia G, Scheller F, Knapp WH, et al. Effects of phosphodiesterase inhibitors on tension induced by norepinephrine and accumulation of cyclic nucleotides in isolated human prostatic tissue. Urology. 2008;71:526–30. doi: 10.1016/j.urology.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K. Sildenafil in the treatment of erectile dysfunction: an overview of the clinical evidence. Clin Interv Aging. 2006;1:403–14. doi: 10.2147/ciia.2006.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy GK, editor. American Hospital Formulary Service Drug Information 2002 Bethesda, MD; American Society of Health System Pharmacists, Inc.2002 (Plus Supplements). p1819 [Google Scholar]
- Kloner RA, Jackson G, Emmick JT, Mitchell MI, Bedding A, et al. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol. 2004;172 5 Pt 1:1935–40. doi: 10.1097/01.ju.0000142687.75577.e4. [DOI] [PubMed] [Google Scholar]


