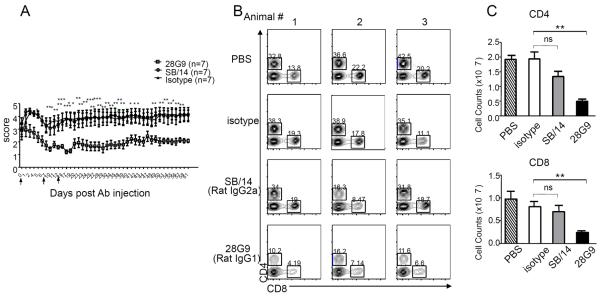
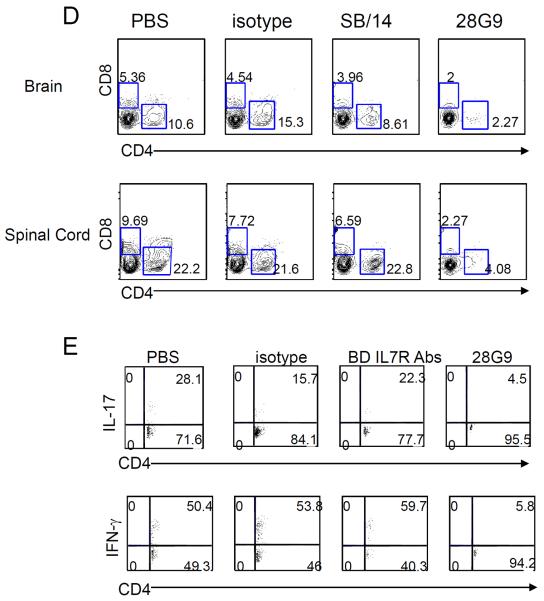
Fig. 6. Anti–IL-7Rα antibody 28G9 showed stronger therapeutic efficacy than SB/14.
(A) Mice that reached clinical scores 2 to 3 were started with 28G9, SB/14, or isotype control once a week for 3 weeks. Dosing was stopped after three injections, and mice were monitored for 7 weeks after injection. Error bars represent mean ± SEM. Statistics were performed with two-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001, n = 7 mice per group). (B and C) CD4+ and CD8+ T cells from mice treated with SB/14 antibody, 28G9 antibody, PBS, or isotype control were analyzed at day 21 after immunization. (B) Percent of CD4+ and CD8+ T cells in the spleen from mice treated with IL-7Rα–specific antibody, 28G9, SB/14, or control. (C) Total CD4+ and CD8+ T cell counts. (D and E) Effects of both IL-7Rα–specific antibodies, SB/14 and 28G9, in CNS. (D) Percentage of CD4+ and CD8+ T cells in the brain (upper) and spinal cord (bottom) from EAE mice treated with SB/14 antibody, 28G9 antibody, PBS, or isotype control analyzed at day 21 after immunization. (E) Percentage of IL-17–producing (upper) and IFN-γ–producing (bottom) CD4+ T cells in the brains of EAE mice treated with IL-7Rα–specific antibody or control. *P < 0.05; **P < 0.01; ***P < 0.001.