Abstract
Human neuroimaging offers a powerful way to connect animal and human research on emotion, with profound implications for psychological science. However, the gulf between animal and human studies remains a formidable obstacle: human studies typically focus on the cortex and a few subcortical regions such as the amygdala, whereas deeper structures such as the brainstem periaqueductal gray (PAG) play a key role in animal models. Here, we directly assessed the role of PAG in human affect by interleaving in a single fMRI session two conditions known to elicit strong emotional responses—physical pain and negative image viewing. Negative affect and PAG activity increased in both conditions. We next examined eight independent data sets, half featuring pain stimulation and half negative image viewing. In sum, these data sets comprised 198 additional participants. We found increased activity in PAG in all eight studies. Taken together, these findings suggest PAG is a key component of human affective responses.
Keywords: periaqueductal gray, emotion, affect, pain, fMRI
INTRODUCTION
Historically, much of what we know about mind, brain and behavior has come from electrophysiological and lesion methods in animals. In the last 15 years, fMRI has emerged as a non-invasive, human counterpart to these traditional approaches. This development greatly enhances the potential for animal and human research to directly inform one another, as homologies across species can be established based on similarities in brain function. Such work is crucial for understanding human brain function, as fMRI can provide only correlational measures of neural activity. Causal inference requires invasive and disruptive methods. While such methods are commonly used in animal research, in humans they are limited and rare.
However, at present there exists a substantial gulf between human and animal work on affective processes, because human and animal studies focus largely on different brain structures. Human fMRI studies have focused primarily on the cerebral cortex and structures such as the amgydala, whereas animal models of emotion focus on deeper subcortical structures, often describing pathways connecting the brainstem to the periphery. Although there is some overlap in basal telencephalic structures such as the amygdala (LeDoux, 2007) and ventral striatum (Cardinal et al., 2002), key players in animal models of emotion, including the midbrain periaqueductal gray (PAG; Bandler and Shipley, 1994; Behbehani, 1995; Panksepp, 1998), hypothalamus (Sewards and Sewards, 2003), and other brainstem nuclei (Alcaro et al., 2007), have been largely absent from models of emotion based on human neuroimaging.
One possible explanation for this discrepancy is that it reflects a true difference between species in the neural bases of emotion. A second possibility is that the human neuroimaging techniques lack sensitivity to reliably detect changes in small, ventral brain regions like those that are prominent in the animal literature. However, while the cortex and amygdala clearly play important roles in affective processes, a recent meta-analysis of human neuroimaging studies of emotion questioned their centrality to emotional experience, finding amygdala activations most reliably reflect salience detection and emotion perception, while rostral anterior cingulate and anterior insula participate extensively in cognitive processes likely unrelated to emotion (Wager et al., 2008a). Furthermore, meta-analytic evidence suggests that human neuroimaging studies do indeed reliably detect emotion-related activity in the brainstem and hypothalamus (Kober et al., 2008; Wager, et al., 2008a). Taken together, previous research suggests that the neural architecture of human emotion may more closely resemble that observed in animal research, and regions such as the brainstem and hypothalamus can be reliably imaged using standard neuroimaging techniques.
In the present research, we chose to focus on the midbrain PAG, an area thought to be central in driving emotional experience and physiology in non-human animals, particularly in response to threat (Bandler and Carrive, 1988; Cezario et al., 2008), as part of the motivational drive for hunting and foraging (Sukikara et al., 2010) and during sexual and maternal behaviors (Salzberg et al., 2002). Across these diverse affective and motivational circumstances, PAG may serve to flexibly coordinate the common and distinct brain regions needed to implement an appropriate set of behavioral, physiological, and experiential responses (Bandler and Shipley, 1994; Behbehani, 1995; Panksepp, 1998).
In spite of this considerable animal literature suggesting PAG involvement in affective and motivational processes beyond nociception, the human neuroimaging literature on emotion seldom has discussed PAG (for exceptions, see: Damasio et al., 2000; Mobbs et al., 2007; Del-Ben and Graeff, 2009; Mobbs et al., 2009; Wager et al., 2009; Mobbs et al., 2010; Linnman et al., 2012). However, a growing literature on PAG activity related to physical pain—a strong elicitor of negative affect—suggests that PAG can be reliably imaged with current standard fMRI sequences (Wager et al., 2004; Kong et al., 2010; Schoell et al., 2010; Linnman et al., 2012). While it is not clear if this PAG activity is directly related to the emotional aspect of pain, recent meta-analyses of human neuroimaging studies found consistent activation of PAG during negative emotional processing unrelated to nociception (Kober et al., 2008; Wager et al., 2008a), suggesting the limited discussion of PAG in the human emotion literature may not reflect a true functional difference between species.
To address this issue, we first conducted an experiment that interleaved phasic heat stimulation and presentation of aversive photographs during a single fMRI session. We chose physical pain and negative image viewing because we have found both reliably increase negative affect. We hypothesized that PAG activity would be greater during both pain and negative image viewing, consistent with animal data demonstrating a broad role for PAG in negative emotion. While pain is an inherently aversive primary reinforcer, images typically require conceptual, social, or memory-guided interpretation in order to evoke emotion. Thus, this study also explores whether PAG is activated even when affective responses are largely conceptually driven, a possibility not easily tested in animal models.
To provide additional, independent tests of our hypothesis, we next examined the area of PAG overlap in eight additional data sets, four of which featured high and low pain and four of which featured negative and neutral images. Altogether, these independent data sets comprised 198 additional participants. Despite heterogeneity in the experimental designs, participant demographics, analysis techniques, and MRI magnets used, we hypothesized we would observe increased activity in PAG in all eight studies. Taken together, these findings would suggest PAG is a core region involved in human emotion.
METHODS
Participants
The initial study included 16 participants [five women; ages 18–45 years, M (s.d.) = 31.75 (5.18)].
Procedure
A standard nociceptive calibration was performed to determine temperature levels needed to evoke similar levels of pain for each participant (Buhle and Wager, 2010).
The task consisted of five functional runs consisting of 24 trials each, for a total of 120 trials. Each trial began with a temporally jittered white fixation cross (4, 5, 6, 7 or 8 s), followed by a 6 s image presentation or thermal stimulation. The images presented consisted of 30 negative and 30 neutral images from the International Affective Picture System (Lang et al., 2008). The thermal stimuli consisted of 30 high and 30 low pain stimulations. During thermal stimulations, a yellow cross appeared on the display. A second temporally jittered white fixation cross (4, 5, 6 or 7 s) followed the stimulus. After this variable interval, the trial concluded with a 4 s continuous rating scale, during which participants used a trackball to indicate how negative they felt about the stimulus, with 0 indicating ‘not at all negative’ and 8 indicating ‘very negative’.
Noxious heat and image stimuli alternated throughout the task, but the level of each stimulus (low/neutral or high/negative) varied randomly. This experiment also featured a cued manipulation of psychological mindset, whereby participants were asked to adopt a reactive mental stance to half the stimuli, and an accepting mental stance to the remaining stimuli. This manipulation is not of interest to the present question and there was no interaction of stimulus level and mindset for either pain or negative images within the PAG, so in all subsequent analyses reported here we collapsed across the two mindsets. The effects of mindset on other brain regions will be reported in a separate paper.
Image acquisition parameters
Participants were scanned in a 1.5 Tesla General Electric Signa Twin Speed Excite HD scanner. Functional images were acquired with a T2*-weighted EPI BOLD ascending interleaved sequence with a TR of 2000 ms, TE of 34 ms, flip angle of 90°, 64 × 64 in-plane matrix, field of view of 22.4 cm, 28 4.5-mm thick slices, yielding a voxel size of 3.5 × 3.5 × 4.5 mm.
Image processing
Standard preprocessing in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) included: slice scan-time correction; realignment and motion correction; anatomical-functional coregistration; normalization to MNI space using Unified Segmentation (Ashburner and Friston, 2005) and resampling to 3 mm3 voxels; and smoothing with a 6-mm Gaussian kernel.
fMRI analyses
fMRI analyses used custom MATLAB software implemented in NeuroElf (http://neuroelf.net/). First-level general linear model analyses used robust regression, with motion parameters and a 400 s high-pass filter as additional regressors of no interest. Next, a whole-brain, second-level, random effects analysis was performed with robust regression (Wager et al., 2005). All results were thresholded at P < 0.05, Familywise Error Rate corrected for cluster extent within gray matter (P < 0.001 and k = 12 contiguous voxels, calculated using NeuroElf’s instantiation of AlphaSim (Forman et al., 1995). An interaction analysis of Pain and Negative Image Viewing identified areas in which the difference was significantly larger or smaller between the Hot and Warm conditions than between the Negative and Neutral conditions. However, it is important to note that the Hot and Negative responses were not necessarily equally potent with respect to their control conditions, so the presence or absence of an interaction would not be conclusive. A conjunction null analysis identified areas of overlap between Pain and Negative Image Viewing within the PAG (Nichols et al., 2005).
Contrast time courses
To confirm that the standard hemodynamic response function (HRF) used in the main analysis fit was appropriate, a deconvolution (finite-impulse-response) regression model was computed to estimate the average, systematic deviation of BOLD response in each TR following the stimulus onset for each of the two contrasts of interest in the area of PAG overlap. The design matrices thus contained one regressor (independent variable) per condition and TR. Given the fact that stimuli were non-TR-locked, BOLD time courses from the area of overlap were first up-sampled to a 0.5 s resolution using cubic spline interpolation. The resulting regression weights were then resampled to a 0.1 s resolution for display purposes. Analysis with the original data yielded similar but less smooth results.
Analysis of independent data sets
To confirm the reliability of PAG involvement in Pain and Negative Image Viewing, we additionally examined fMRI data from eight previously conducted experiments (Wager et al., 2008b; Atlas et al., 2010; McRae et al., 2010; Kross, et al., 2011; McRae et al., 2012). Four of these additional data sets (Studies 1–4) featured Pain [high > low thermal pain] contrasts, while the other four featured Negative Image Viewing [negative > neutral image] contrasts (Studies 5–6). These data sets varied in a number of ways, including sample and stimulus characteristics, and collection site. None of the contrasts analyzed featured manipulations of psychological mindset. In Study 1, participants were simply asked to think about the sensations they experienced. In Study 2, valid cues (high vs low) preceded each trial. In Studies 5–6, participants were asked to look the images and respond naturally. In Study 1, and in Studies 5–8, participants rated negative affect after each trial. In Studies 2–4, participants rated pain after each trial. Additional Information about each data set is summarized in Table 1. One-tailed, independent-sample t-tests were performed on the average extracted contrast values in the PAG overlap region of interest (ROI) identified in the initial experiment.
Table 1.
Summary information on eight independent data sets analyzed to test the reliability of the findings from the present study that both Pain and Negative Image Viewing activate the PAG
| Study | Modality | N (female) | Age, mean (s.d.) | Stimulus duration, (s) | Number of stimuli | Collection site | Scanner and sequence | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Pain | 40 (21) | 20.78 (2.59) | 15 | 16 | Columbia | 1.5 T GE, Spiral I/O | Kross et al., 2011 |
| 2 | Pain | 18 (9) | 25.5 (5.8) | 10 | 32 | Columbia | 1.5 T GE, Spiral I/O | Atlas et al., 2010 |
| 3 | Pain | 20 (8) | 28.8 (7.5) | 10 | 24 | Columbia | 1.5 T GE, Spiral I/O | L.Y. Atlas et al., manuscript under review |
| 4 | Pain | 20 (10) | 22.05 (3.48) | 10 | 24 | Columbia | 1.5 T GE, Spiral I/O | L.Y. Atlas et al., manuscript under review |
| 5 | Images | 38 (21) | 16.47 (3.82) | 8 | 16 | Stanford | 3.0 T GE, Spiral I/O | McRae et al., 2012 |
| 6 | Images | 30 (18) | 21.97 (4.56) | 8 | 72 | Columbia | 1.5 T GE, EPI | Wager et al., 2008b |
| 7 | Images | 14 (8) | 35.43 (10.96) | 8 | 56 | Stanford | 1.5 T GE, Spiral I/O | K.N. Ochsner et al., Unpublished |
| 8 | Images | 18 (18) | 24.4 (3.5) | 8 | 36 | Stanford | 1.5 T GE, Spiral I/O | McRae et al., 2010 |
For purposes of visualization, whole-brain contrast maps were also created using the same threshold as in initial experiment (P < 0.001 uncorrected and k = 12, P < 0.05 cluster extent-corrected in the initial experiment). For Study 4, this combination of height and extent thresholds did not reveal any activity within the PAG. A second simulation indicated that a corrected threshold of P = 0.05 would be obtained through the combination of an uncorrected threshold of P < 0.005 and a cluster threshold of k = 85 3 × 3 × 3 mm3 voxels. This corrected threshold revealed activity in the PAG and was thus used to display results for Study 4.
RESULTS
Initial experiment
In-scan ratings of negative effect
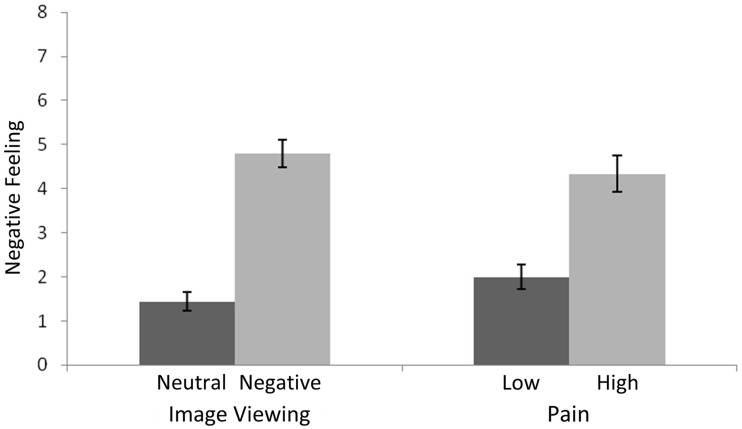
Participants reported greater negative affect for the aversive/high stimuli than for the neutral/low stimuli, F(1,60) = 81.99, MSE = 129.58, P < 0.001, but similar negative affect for the image and pain stimuli, F(1,60) = 0.03, MSE = 0.04, P = 0.87 (Figure 1). There was no interaction between stimulus type and affective intensity, F(1,60) = 2.60, MSE = 4.11, P = 0.11.
Fig. 1.
Affect ratings for pain and image stimuli. Error bars reflect between-subject standard error.
fMRI
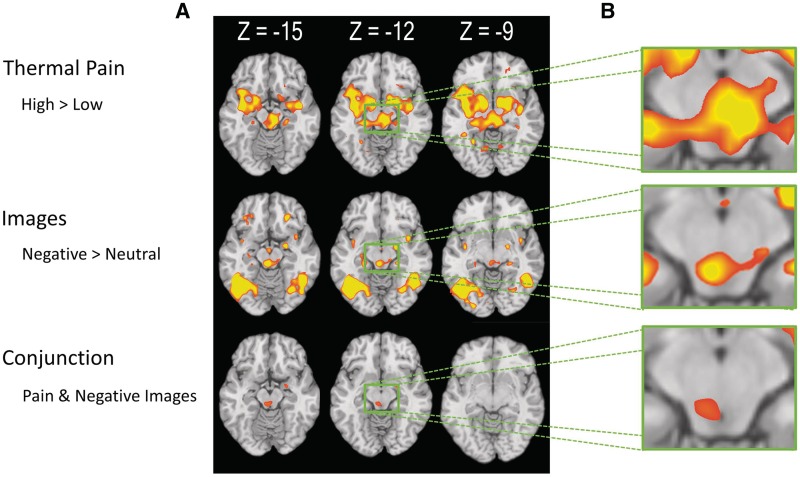
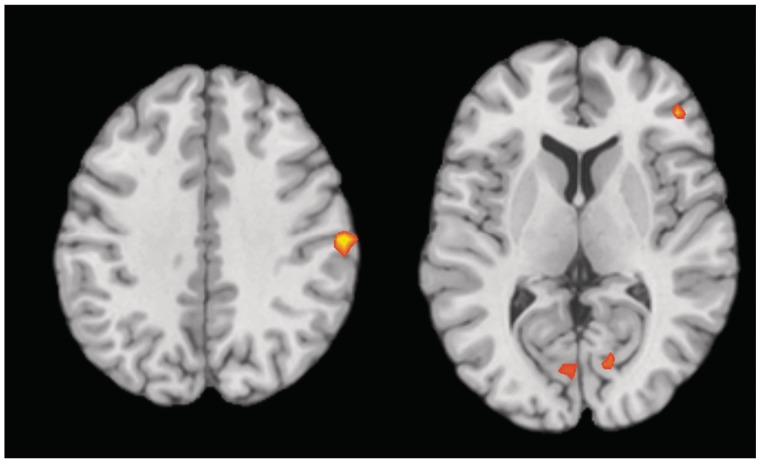
Whole-brain contrasts of both high vs low pain and negative vs neutral image viewing revealed activity in PAG (Figure 2). No interaction of Pain and Negative Image viewing was found in or near the PAG, indicating that the magnitude of the contrast activity did not reliably differ between the conditions. A conjunction null analysis of these contrasts within the PAG mask delineated an area of overlap (coordinates = −3, −30, −15; k = 17; Figure 2). Other regions involved in both conditions included right inferior parietal lobe, right inferior frontal gyrus, right amygdala, and bilateral cuneus (Figure 3 and Table 2).
Fig. 2.
PAG activity associated with pain and negative image viewing. (A) Whole-brain axial slices. The first row shows areas in which activity was greater in the High compared to the Low Pain condition. The second row shows areas in which activity was greater in the Negative compared to neutral image viewing condition. The third row shows the conjunction of these two contrasts. (B) Detail of PAG at z = −12.
Fig. 3.
Axial slices showing activity associated with Pain and Negative Image Viewing. The first slice (z = 36) shows a cluster in right inferior parietal lobe. The second slice (z = 6) shows clusters in bilateral cuneus and right inferior frontal gyrus.
Table 2.
Overlap of Pain and Negative Image Viewing in initial experiment
| Region | Peak coordinate | Cluster size | Peak t-value |
|---|---|---|---|
| Inferior parietal lobe | 63, −18, 36 | 30 | 6.52 |
| Inferior frontal gyrus | 45, 39, 6 | 15 | 5.5 |
| Amygdala | 24, −6, −15 | 13 | 4.66 |
| PAG | −6, −30, −15 | 17 | 4.63 |
| Cuneus | 15, −72, 3 | 22 | 4.44 |
| Cuneus | −6, −78, 3 | 16 | 4.39 |
Peak coordinates given in MNI space (X, Y, Z).
Contrast time courses
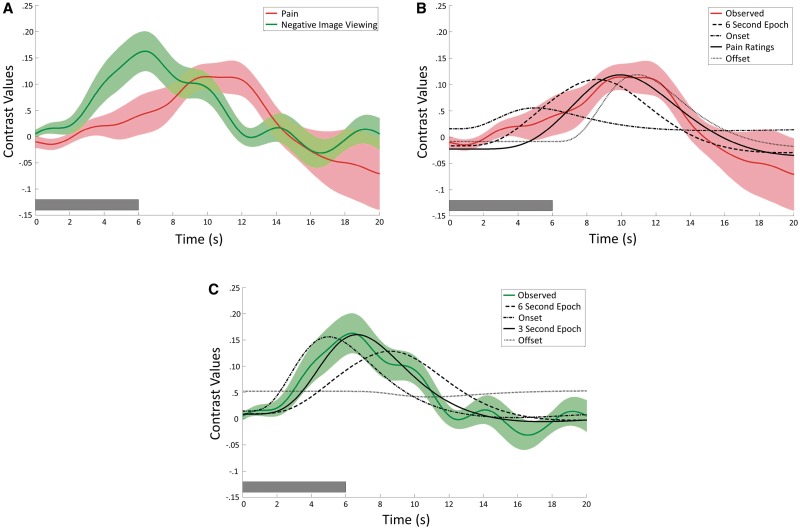
Deconvolution (finite-impulse-response) regression time course plots for both the Pain and Negative Image Viewing contrasts showed typical hemodynamic response characteristics, including a significant, positive peak and an expected delay from onset (Figure 4). The plots reveal an apparent difference in peak latency, with the Pain contrast peak occurring subsequent to that of Negative Image Viewing. This difference in time-to-peak is consistent with differences in the inherent nature of the heat and pictorial stimuli. That is, while images are rapidly processed, the heat stimuli required a ramp up time of ∼2 s to reach peak temperature, and peak pain would occur still later, due to temporal summation. Panels B and C depict the show different plausible stimulus models overlaid on the time courses for Pain and Negative Image Viewing, respectively. For the Pain contrast, visual inspection suggests that a model based on a typical pain rating trajectory (based on unpublished data collected in our lab) provides a better fit that a 6-s duration, onset only, or offset only model. For the Negative Image Viewing contrast, visual inspection suggests that model based on a 3-s stimulus duration provides a better fit than a 6-s duration, onset only, or offset only model.
Fig. 4.
(A) Deconvolution (finite-impulse-response) random-effects plots of the time courses of the Pain (red) and Negative Image Viewing (green) contrasts in area of PAG overlap identified in the initial experiment (see conjunction analysis in Figure 2). Both time courses show typical HRF characteristics. Semi-transparent bands around the plotted curves represent standard error across subjects. (B) Convolved HRF fits laid over Pain time course. Visual inspection suggests that a model based on a typical pain rating trajectory (based on unpublished data collected in our lab) provides a better fit that a 6-s duration, onset only, or offset only models. (C) Convolved HRF fits laid over Negative Image Viewing time course. Visual inspection suggests that assuming a 3-s stimulus duration provides a better fit than a 6-s duration, onset only, or offset only models. Although the actual stimulus duration was 6 s, it is likely that affective processing declines after the initial few seconds of observation. In all plots, dark bars in lower left corner represent stimulus duration.
Independent data sets
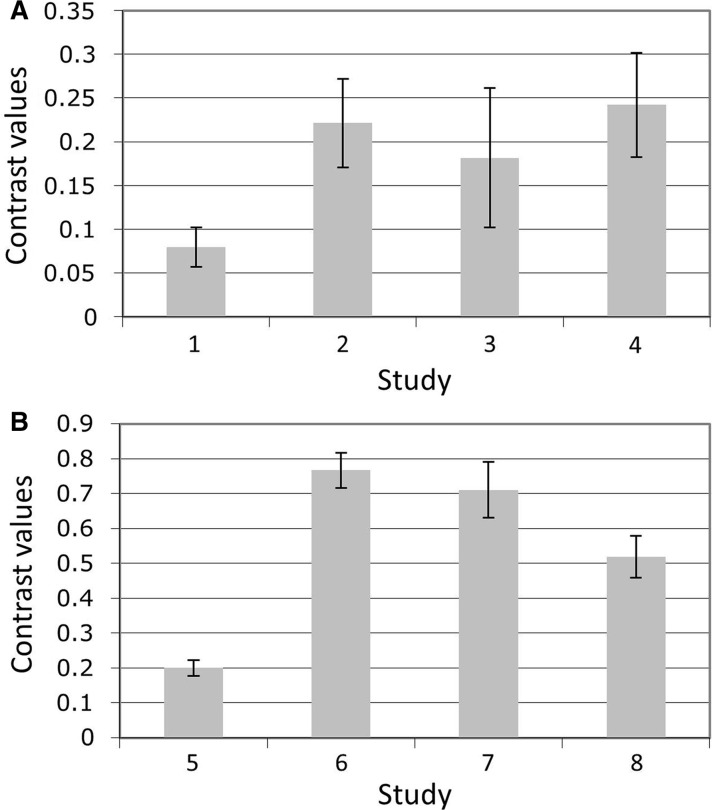
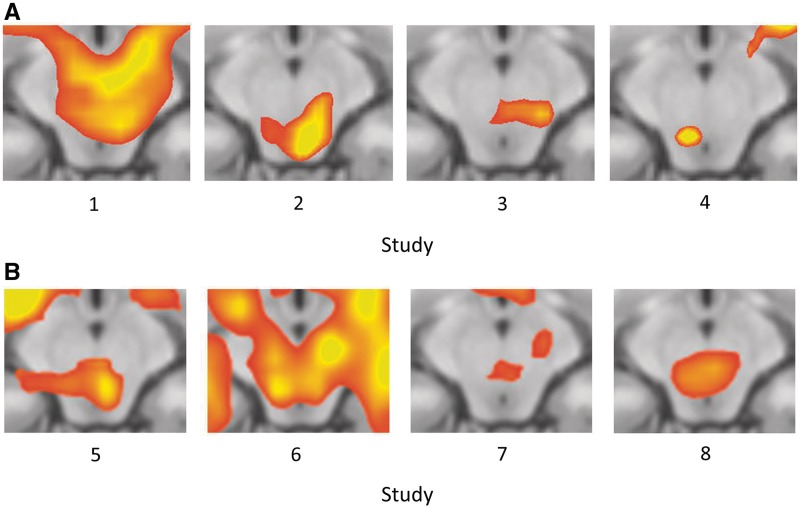
After identifying the PAG overlap region in the present experiment, we performed one-tailed t-tests on the average contrast values in each of eight independent experiments. These tests revealed greater activity in response to high vs low pain in Study 1, t(39) = 3.52, P < 0.001, Study 2, t(17) = 4.36, P < 0.0005, Study 3, t(19) = 2.27, P < 0.02, and Study 4, t(19) = 4.06, P < 0.0005, and to negative vs neutral images in Study 5, t(37) = 4.42, P < 0.00005, Study 6, t(29) = 3.69, P < 0.001, Study 7, t(13) = 2.99, P < 0.01, and Study 8, t(17) = 2.39, P < 0.02 (Figure 5). Additionally, in each of the eight independent data sets, whole-brain contrasts revealed activity in or very close to the PAG (Figure 6).
Fig. 5.
Bar graphs of average extracted contrast values in independent data sets in area of PAG overlap identified in initial experiment (see conjunction analysis in Figure 2). (A) High > Low Pain contrasts from Studies 1–4. (B) Negative > Neutral Image Viewing contrasts from Studies 5–8. All contrasts were significant at P > 0.05, using one-tailed, independent-sample t-tests. Error bars represent between-subjects standard error. Additional information on the data sets is provided in Table 1.
Fig. 6.
PAG activity in eight independent data sets. Images show detail from axial slice of whole-brain contrast at z = −12. (A) High > Low Pain contrasts from Studies 1–4. (B) Negative > Neutral Image Viewing contrasts from Studies 5–8. All clusters are significant at P = .05, corrected (for Study 4, P = 0.005 uncorrected and k = 85; for all others, P = 0.001 uncorrected and k = 12). Additional information on the data sets is provided in Table 1.
DISCUSSION
Both human and animal research show that PAG is involved in pain processing, but the animal literature describes a broader role for PAG in emotional behavior that is seldom acknowledged in the human literature. In the initial experiment, we found that two strongly aversive conditions—physical pain and viewing negative images—increased both negative affect and PAG activity, supporting a broad role for PAG in human negative affect. A conjunction analysis revealed an area of overlap between the two conditions within PAG, and deconvolution (finite-impulse-response) plots of the time courses showed a plausible, positive shape for each contrast. To confirm these results, we examined activity in PAG in eight independent data sets, four of which featured thermal pain and four of which featured negative image appraisal. In each independent data set, whole brain-analyses identified activity clusters in PAG, and ROI analyses using the area of overlap identified in the initial experiment showed greater activity related to pain and negative image viewing. Taken together, these results support the hypothesis that PAG plays an important role in human negative affect, in line with previous evidence from research in animals (Bandler and Shipley, 1994; Behbehani, 1995; Panksepp, 1998). Furthermore, our findings indicate that PAG responds not only to inherently aversive, primary reinforcers such as pain, but to negative emotional experiences that require from conceptual, social, or memory-guided interpretations. More broadly, the present study suggests that human research on the neural substrates of emotion should broaden its purview from the current primary focus on the cerebral cortex and structures such as the amgydala, placing greater emphasis on deeper subcortical and brainstem structures.
One possible concern in fMRI analyses is that contrast differences can be found even when the actual response shapes do not correspond to known hemodynamic behavior. For example, data artifacts may cause spikes in the BOLD response, leading to inflated statistical values. To ensure that the results of the present experiment were not influenced by such artifacts, we examined deconvolution (finite-impulse-response) plots of the average time course for each contrast in the area of overlap. As seen in Figure 4, the shape of each time course was positive and consistent with the canonical HRF. Notably, the peak of the pain time course appears to be delayed relative to that associated with image viewing. In most cases, participants would likely be able to comprehend the content of the images used here within the first second following stimulus onset, and emotional reaction would quickly follow. In contrast, the heat stimuli required a ramp time of ∼2 s to reach the target temperature, and, due to temporal summation, maximum subjective pain occurs at the end of the stimulation period. Thus, these peak differences provide additional assurance that these responses are veridical, and suggest that PAG activity approximately tracks reported pain and potentially experienced negative affect during picture viewing (though affective chronometry was not directly assessed).
Another possible concern in fMRI analyses is whether sequential processes are adequately distinguished. For example, in the present study, relief might reliably follow stimulus offset, potentially confounding stimulus-related activity. Although a strong test of this possibility would require modeling stimulus offset in the context of a design that varied stimulus duration, we explored this possibility in the present data by overlaying the observed time courses with plausible convolved HRFs for both the Pain and Negative Image Viewing contrasts (Figure 4B and C, respectively). For Negative Image Viewing, visual inspection suggests that model based on a 3-s stimulus duration provides a better fit than a 6-s duration, onset only, or offset only model. Thus, the present data do not suggest that relief drives BOLD activity in the PAG. Although the actual stimulus duration was 6 s, it is likely that affective processing declines after the initial few seconds of image viewing. For the Pain contrast, visual inspection suggests that a model based on a typical pain rating trajectory (based on unpublished data collected in our lab) provides a better fit that a 6-s duration, onset only, or offset only model. Although the present data do not suggest that relief drives PAG activity, future work should systematically test for this possibility by varying pain duration and modeling pain offset.
An inherent limitation of neuroimaging is that it provides only correlational data of brain function. While the present data demonstrates that PAG is active during the two aversive conditions of Pain and Negative Image Viewing, we can only speculate at what underlying processes this activity represents. Future fMRI research can help constrain our speculation about these underlying processes by further testing the specificity of the PAG response. For example, it will be important for future work to provide additional tests of the hypothesis that PAG activity is generally involved in negative emotional processing by examining the response to other aversive stimuli, and also to test alternate hypotheses that PAG is involved in both positive and negative emotional processes. Thus far, several studies have reported PAG activity in response to a number of negative emotional conditions, including listening to unpleasant sounds (Zald and Pardo, 2002), social rejection (Eisenberger et al., 2007) and threat and fear (Mobbs et al., 2007, 2009; Mobbs et al., 2010). Additionally, a few studies have reported PAG activity in response to positive emotion stimuli, including pleasant music (Blood and Zatorre, 2001), positive words (Maddock et al., 2003), and images of one’s baby (Noriuchi et al., 2008). Taken together, these findings suggest that PAG may be involved in both positive and negative emotional processes, raising the possibility that the specific underlying functions of PAG may not be exclusively emotional processes, but instead (or in addition) may involve non-emotional cognitive processes related to attention or salience, or non-cognitive physiological functions (Linnman et al., 2012).
However, additional neuroimaging can only constrain the problem space by providing additional correlation data. Only disruptive methods allow one to conclude that an area is causally involved in a behavior or experience. Such methods are common in animal research, and have led too much of our current understanding of PAG behavior. However, animals cannot directly report on their experience. Although quite rare in humans, a small number of studies have documented reports of subjective experience following direct stimulation of PAG. An early study found that PAG stimulation induced diffuse pain, the urge to urinate, and, in one participant, fear so unpleasant that she would not tolerate additional stimulation (Nashold et al., 1969). More recent studies have reported nausea, fright and piloerection (Hosobuchi, 1987); distress, anxiety and weeping (Tasker, 1982); and feelings of apprehension and ‘impending doom’ (Young et al., 1985). The diversity of negative affective responses to PAG stimulation is striking, and provides converging evidence that PAG plays a causal role in negative affect.
Extensive animal research indicates that distinct subregions of PAG subserve specific nociceptive and affective processes. In general, the dorsolateral and lateral PAG have been associated with active emotional coping strategies, including fight-or-flight responses and hypertension, while the ventrolateral PAG has been associated with passive coping strategies, including reduced reactivity and hypotension (Bandler and Shipley, 1994; Behbehani, 1995), although some have challenged the specificity of these divisions (Heinricher and Ingram, 2008). Unfortunately, the spatial resolution of the current data precludes such fine-grained analysis. Looking across the results of the current experiment and the eight independent data sets, it is clear that the peak of activity appears to vary within PAG. This apparent variability likely reflects the spatial resolution of the data, as well as differences in structure–function overlap in the participants and the preprocessing and first-level analysis methods used. In fact, a recent meta-analysis found nearly identical mean coordinates for emotion- and pain-related peaks in PAG (Linnman et al., 2012). Thus, while we can conclude that PAG responds to both pain and negative images, at present we must remain agnostic regarding the precise locations of these responses. Intriguingly, in the majority of the contrasts the peaks appear to fall in the ventral portion of PAG, rather than the dorsolateral and lateral portions that have been more closely associated with aversive behavior. Future work in humans could use high-field scanners (Linnman et al., 2012), spatially optimized acquisition protocols (Napadow et al., 2009), brainstem-specific methods for normalization (Napadow et al., 2006) and removal of cardiac-related distortions (Guimaraes et al., 1998; Napadow et al., 2008), in order to obtain PAG data with greater spatial resolution, so that more fine-grained comparison can be made between the response to negative affective stimuli in human PAG and the subregions delineated in animal research. Given the rich animal literature on emotional processing in other brainstem structures, such methods would also be important for establishing additional homologies between animal and human models.
Although our primary goal in this study was to examine the response of PAG to physical pain and negative images, the conjunction analysis revealed a number of other regions involved in both conditions, including right inferior parietal lobe, right inferior frontal gyrus, right amygdala and bilateral cuneus (Table 2). The amygdala is well known for its involvement in the response to salient, emotional stimuli (LeDoux, 2007; Sergerie et al., 2008) and reciprocal projections link it with PAG (Rizvi et al., 1991; An et al., 1998; Pereira et al., 2010). Inferior frontal gyrus is thought to play either a specific role in inhibitory control (Aron et al., 2004), or possibly a more general role in attentional control and the response to salient and behaviorally relevant stimuli (Hampshire et al., 2010). The inferior parietal activation occurred in an area that is typically associated with movement, but in the present study participants did not make responses until after the stimulus period, and responses were made with the right hand, which should have resulted in contralateral activity. However, this region is adjacent to secondary somatosensory cortex, an area that has been associated both with pain experience, and with the observation or imagining of pain in one’s self and others (Jackson et al., 2006; Budell et al., 2010; Kross et al., 2011). Many of the negative images we used depicted people in physical pain or otherwise suffering, possibly accounting for our observation of this region during negative image viewing. Cuneus activity is most often associated with visual processing, and increased attention to emotional images may have increased visual processing for the negative compared to the neutral images. Interestingly, cuneus activity also been linked to the experience of thirst (Egan et al., 2003; Farrell et al., 2006) and to the affective dimension of pain (Fulbright et al., 2001; Matharu et al., 2004), suggesting a role in non-visual aversive processing akin to what we observed.
CONCLUSION
To our knowledge, this study provides the first within-subject confirmation of a shared role for PAG in pain and non-pain-related negative emotion in humans. Moreover, we replicated these results in eight independent data sets comprising 198 additional participants. Viewed alongside the extensive animal literature, these findings suggest that PAG function in affect is conserved across species, and that human PAG may be a core region in the generation of negative emotion. Future work in humans should build on these findings, using high-resolution methods to compare PAG response to different types of affective stimuli, and to explore other functional homologies suggested by the rich animal literature on emotional processing in deeper subcortical and brainstem structure.
Conflict of Interest
None declared.
Acknowledgments
J.T.B., H.K., K.N.O., B.L.H., E.K. and T.D.W. designed the experiment. J.T.B., P.M.S. and H.K. collected data. J.T.B., H.K., P.M.S., J.W. and T.D.W. analyzed the data. J.T.B. and T.D.W. wrote the article. Independent dataset collection and analysis was done by J.W., L.Y.A. and K.M. This research was supported by the National Institute of Mental Health [grant numbers NIH MH076137, awarded to K.N.O. and NIH MH076136, NIH RC1DA028608 and NIH R01DA027794, awarded to T.D.W.]; the National Institute of Drug Addiction [grant number NIDA DA022541, awarded to K.N.O.; NSF 0631637]; and the Mind and Life Institute, through a 2005 Mind and Life Summer Research Institute Francisco J. Varela Memorial Grant Award to B.L.H. and Diego E. Berman.
REFERENCES
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Research Reviews. 2007;56(2):283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. Journal of Comparative Neurology. 1998;401(4):455–79. [PubMed] [Google Scholar]
- Aron A, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30(39):12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Research. 1988;439(1–2):95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in Neurosciences. 1994;17(9):379–89. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Progress in Neurobiology. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11818–23. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budell L, Jackson P, Rainville P. Brain responses to facial expressions of pain: emotional or motor mirroring? Neuroimage. 2010;53(1):355–63. doi: 10.1016/j.neuroimage.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149(1):19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cezario AF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Hypothalamic sites responding to predator threats—the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. European Journal of Neuroscience. 2008;28(5):1003–15. doi: 10.1111/j.1460-9568.2008.06392.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009:108–35. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan G, Silk T, Zamarripa F, et al. Neural correlates of the emergence of consciousness of thirst. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15241–6. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7(4):745–54. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Egan GF, Zamarripa F, et al. Unique, common, and interacting cortical correlates of thirst and pain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2416–21. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Troche CJ, Skudlarski P, Gore JC, Wexler BE. Functional MR imaging of regional brain activation associated with the affective experience of pain. American Journal of Roentgenology. 2001;177(5):1205–10. doi: 10.2214/ajr.177.5.1771205. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Melcher JR, Talavage TM, et al. Imaging subcortical auditory activity in humans. Human Brain Mapping. 1998;6(1):33–41. doi: 10.1002/(SICI)1097-0193(1998)6:1<33::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher HM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses: A Comprehensive Reference. Vol. 5. San Diego: Academic Press; 2008. pp. 593–626. [Google Scholar]
- Hosobuchi Y. Dorsal periaqueductal gray-matter stimulation in humans. Pacing and Clinical Electrophysiology. 1987;10(1 Pt 2):213–6. doi: 10.1111/j.1540-8159.1987.tb05951.x. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148(2):257–67. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(15):6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville: University of Florida; 2008. [Google Scholar]
- LeDoux JE. The amygdala. Current Biology. 2007;17(20):R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage. 2012;60(1):505–22. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127(Pt 1):220–30. doi: 10.1093/brain/awh022. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, et al. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents, and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, et al. From threat to fear: the neural organization of defensive fear systems in humans. Journal of Neuroscience. 2009;29(39):12236–43. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–83. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20582–6. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42(1):169–77. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Kennedy D, Hui KK, Makris N. Automated brainstem co-registration (ABC) for MRI. Neuroimage. 2006;32(3):1113–9. doi: 10.1016/j.neuroimage.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Park K, et al. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. Neuroimage. 2009;47(1):289–301. doi: 10.1016/j.neuroimage.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. Journal of Neurosurgery. 1969;30(1):14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biological Psychiatry. 2008;63(4):415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience : The Foundations of Human and Animal Emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Pereira EA, Lu G, Wang S, et al. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Experimental Neurology. 2010;223(2):574–81. doi: 10.1016/j.expneurol.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. Journal of Comparative Neurology. 1991;303(1):121–31. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Salzberg HC, Lonstein JS, Stern JM. GABA(A) receptor regulation of kyphotic nursing and female sexual behavior in the caudal ventrolateral periaqueductal gray of postpartum rats. Neuroscience. 2002;114(3):675–87. doi: 10.1016/s0306-4522(02)00358-5. [DOI] [PubMed] [Google Scholar]
- Schoell ED, Bingel U, Eippert F, et al. The effect of opioid receptor blockade on the neural processing of thermal stimuli. PLoS One. 2010;5(8):e12344. doi: 10.1371/journal.pone.0012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):811–30. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Research Bulletin. 2003;61(1):25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behavior Brain Research. 2010;209(2):226–33. doi: 10.1016/j.bbr.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Tasker RR. Identification of pain processing systems by electrical stimulation of the brain. Human Neurobiology. 1982;1(4):261–72. [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The Handbook of Emotions. 3rd edn. New York: Guilford; 2008a. pp. 249–71. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008b;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47(3):836–51. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RF, Kroening R, Fulton W, Feldman RA, Chambi I. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. Journal of Neurosurgery. 1985;62(3):389–96. doi: 10.3171/jns.1985.62.3.0389. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16(3 Pt 1):746–53. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]