FIGURE 1.
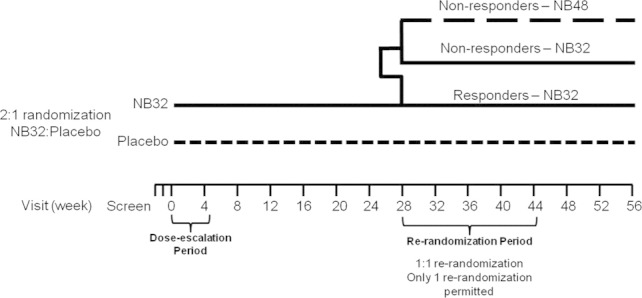
Following screening, participants were randomized via a centrally administered interactive voice response system in a 2:1 ratio, stratified by study site, to receive a combined oral formulation of 32 mg/day naltrexone SR + 360 mg/day bupropion SR (NB32) or matching placebo, administered in divided doses twice daily. Naltrexone was initiated at one-eighth or one-quarter of the maintenance dose and bupropion was initiated at one-quarter of the maintenance dose; doses were escalated linearly over the first 3-4 weeks, and the maintenance dose was reached by the start of week 5. To evaluate the efficacy and safety of a dose increase in participants with suboptimal response, NB32 participants with <5% weight loss at visits between weeks 28 and 44 inclusive were re-randomized (double-blind, 1:1 ratio) to continue receiving NB32 or escalate to NB48 (48 mg/day naltrexone SR + 360 mg/day bupropion SR) for the remainder of the study. Study visits occurred at baseline (week 0) and every 4 weeks thereafter.
