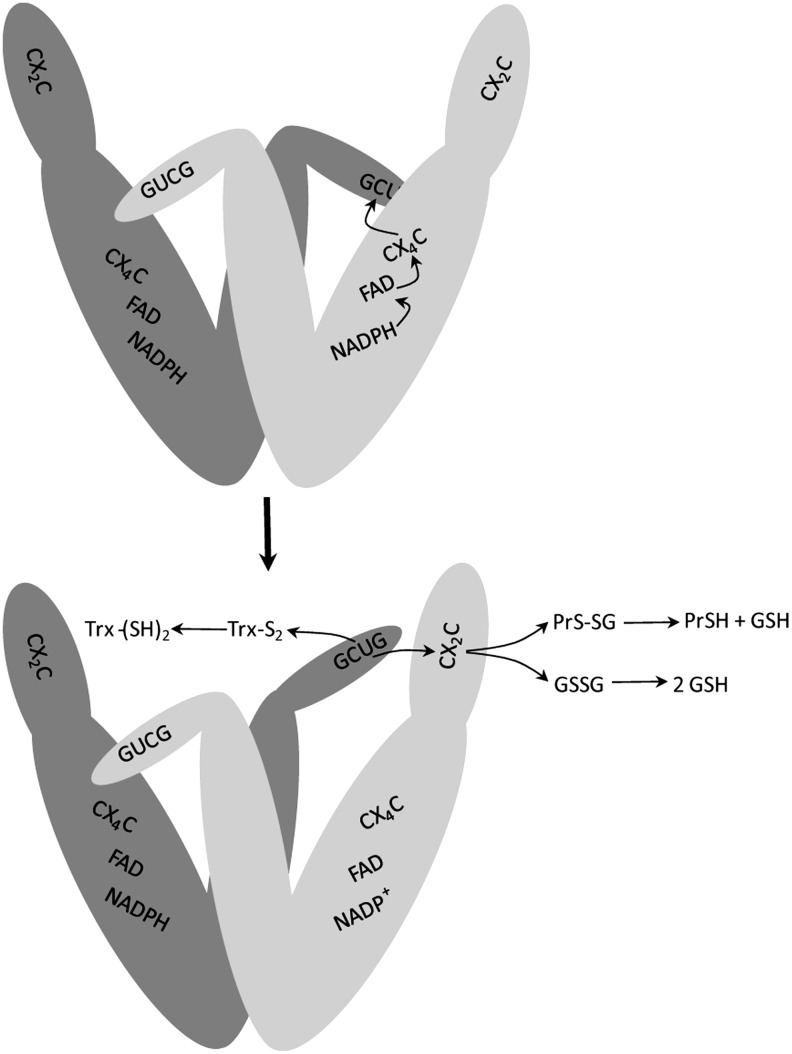
FIG. 4.
Model of the proposed reaction mechanism of TGR. The active form of TGR is a dimer of identical subunits. In the upper part, the flow of reducing equivalents in the TR module of TGR is shown. In the lower part, reduction of the GCUG redox center produces a structural change in the flexible carboxy-terminal arm allowing it to be repositioned to either react with the glutaredoxin domain active site (CX2C) or directly with oxidized Trx (Trx-S2). The reduced glutaredoxin domain can then interact with its substrates, glutathione disulfide (GSSG) or glutathionylated proteins (PrS-SG). Adapted from ref. (41).