FIG. 7.
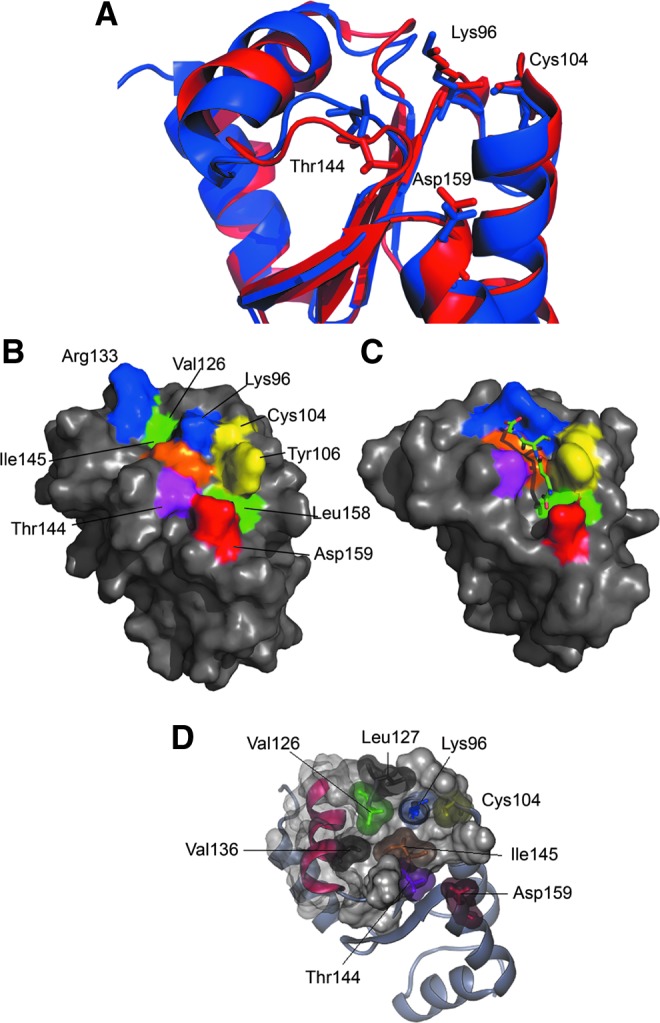
Structural features of the Tb1-C-Grx1 active-site region. (A) Superposition of the protein portion encompassing the putative GSH-binding pocket in apo-Δ76 Tb1-C-Grx1 (red, this work) and E. coli Grx4 in an ISC-bound dimeric form (blue; PDB-ID 2WIC; a single monomer is shown, and the ISC and GSH molecules are omitted). (B) Conserved residues predicted to be important for the binding of GSH are mapped on the surface of Δ76 Tb1-C-Grx1 with different colors: Lys96 and Arg133 in blue; Cys104 and Tyr106 in yellow; Thr144 in magenta; Ile145 in orange; and Asp159 in red; the hydrophobic residues Val126 and Leu158 in the pocket are shown in green. (C) The corresponding residues in E. coli Grx4 (PDB ID 2WIC) are represented with the same colors; the noncovalently bound GSH molecule is shown as sticks. The program PyMol was used for preparing the pictures. (D) Residues Ile145, Val 136, Val126, and Leu127, forming the hydrophobic network discussed in the text, are represented by their van der Waal's surfaces. Other residues important for GSH binding are highlighted with the same color used in panel (B) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
