Abstract
Significance: In the single mitochondrion of protozoan trypanosomatid parasites there are several sites for the generation and elimination of reactive oxygen species (ROS), a class of molecules that exhibit a dual role in cells, either as regulatory mediators or as cytotoxic effectors. Recent Advances: Formation of ROS in trypanosomatid mitochondria can be induced by various drug compounds. Importantly, it can also be triggered by specific physiologic stimuli, indicating that this phenomenon may occur in living parasites as well. Elimination of ROS in these organelles is attributed to the activity of two iron-dependent superoxide dismutases (FeSODs) and up to three different peroxidases (a cytochrome c peroxidase and two thiol peroxidases). Critical Issues: Data regarding the formation of ROS in trypanosomatid mitochondria are limited and nonsystematic. Another critical issue refers to the exact contribution of mitochondrial FeSODs and peroxidases for ROS removal, given that their antioxidant activity is not essential when abrogated individually. This suggests some level of functional overlapping or that ROS produced in mitochondria under normal conditions can be removed noncatalytically. Also still unsolved is the mechanism by which mitochondrial thiol peroxidases are regenerated to their reduced (active) form. Future Directions: The production of intramitochondrial ROS under physiologic conditions and their implication in parasite biology should be further clarified. The relative importance of enzymatic versus nonenzymatic mechanisms for ROS elimination in trypanosomatid mitochondria also requires investigation. Simultaneous depletion of several redundant antioxidant enzymes and determination of noncatalytic antioxidants are possible ways to achieve this. Antioxid. Redox Signal. 19, 696–707.
Introduction
Mitochondria are organelles where essential physiologic processes take place. The hallmark of these is oxidative phosphorylation, which provides aerobic organisms the majority of their energy, but other important functions, namely the synthesis and catabolism of crucial amino acids, fatty acid oxidation, or iron–sulfur cluster biogenesis, are ascribed to these compartments. Mitochondria are also organelles where reactive oxygen species (ROS) (i.e., free radicals and other molecules derived from the incomplete one-electron reduction of molecular oxygen) can be found (50, 51), either because they are generated there or because they diffuse into this organelle from other cell sites. Although fluctuations in the basal levels of ROS in response to certain stimuli do occur and are crucial for cell physiology (10), high concentrations induce oxidative stress and need to be removed in order to prevent toxicity. This review contemplates mitochondrial redox metabolism, focusing on the production of ROS and on their elimination in mitochondria of trypanosomatid parasites.
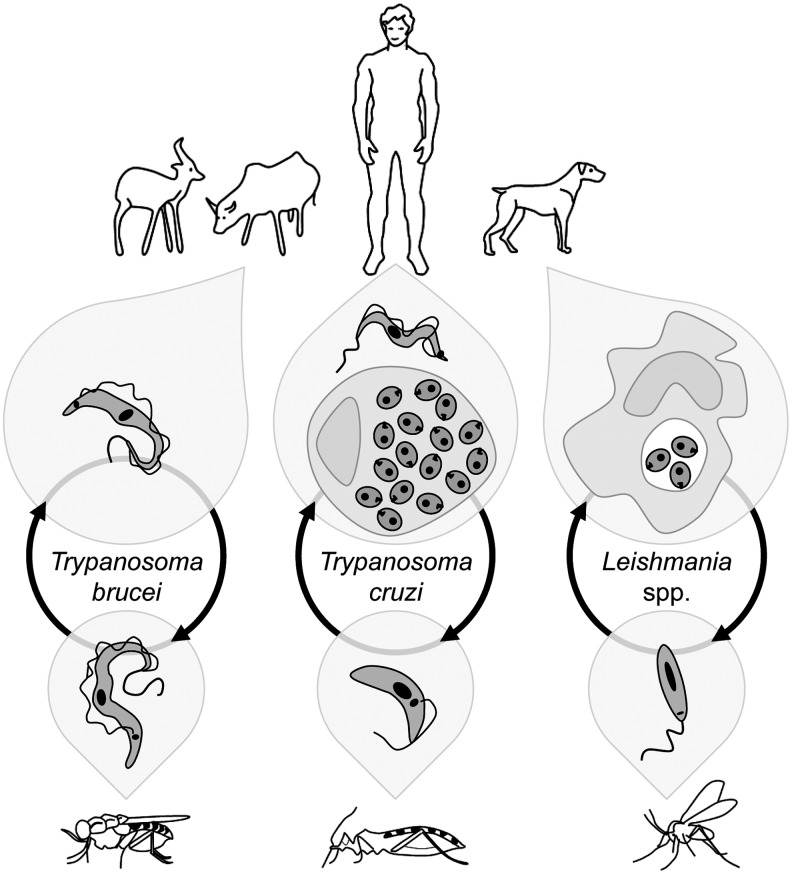
Trypanosomatids encompass a vast group of organisms included in the order Kinetoplastida, many of which are parasites of humans, animals, and plants. For simplicity, this review is restricted to the medically relevant Leishmania spp., the agents of human and canine leishmaniasis, to the Trypanosoma brucei complex, which causes sleeping sickness in humans and Nagana in cattle, and to Trypanosoma cruzi, responsible for Chagas' disease. The main life cycle stages of these parasites are depicted in Figure 1.
FIG. 1.
Life cycles of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania spp. Trypanosomatids have digenic life cycles, which alternate between an insect vector, usually responsible for disease transmission, and a mammalian host. In the invertebrate hosts, trypanosomatids infect the gut, wherein they adopt a flagellated form and an extracellular life style. These insect vector stages are designated as procyclic (T. brucei), epimastigote (T. cruzi), or promastigote (Leishmania spp.). In the case of the mammalian stages of these organisms, their morphology and residence varies according to the parasite species. Trypanosoma brucei resides as a flagellated, extracellular cell (the trypomastigote or bloodstream form) in the circulatory and lymphatic systems of its vertebrate hosts. As for T. cruzi, it alternates between an extracellular, flagellated, and nonreplicative trypomastigote form, and an intracellular, aflagellated, and replicative amastigote stage. Almost any cell, phagocytic or nonphagocytic, can be infected by T. cruzi, although the parasite has a tropism for muscle and nervous cells. Finally, Leishmania spp. resides and replicates as nonmotile amastigotes inside the phagolysosomes of macrophages. Also depicted are a tse-tse fly, a triatomine bug, and a sandfly, the insect vectors for T. brucei, T. cruzi, and Leishmania spp., respectively. These organisms cause diseases in humans and also in cattle (T. brucei) and in dogs (Leishmania spp.).
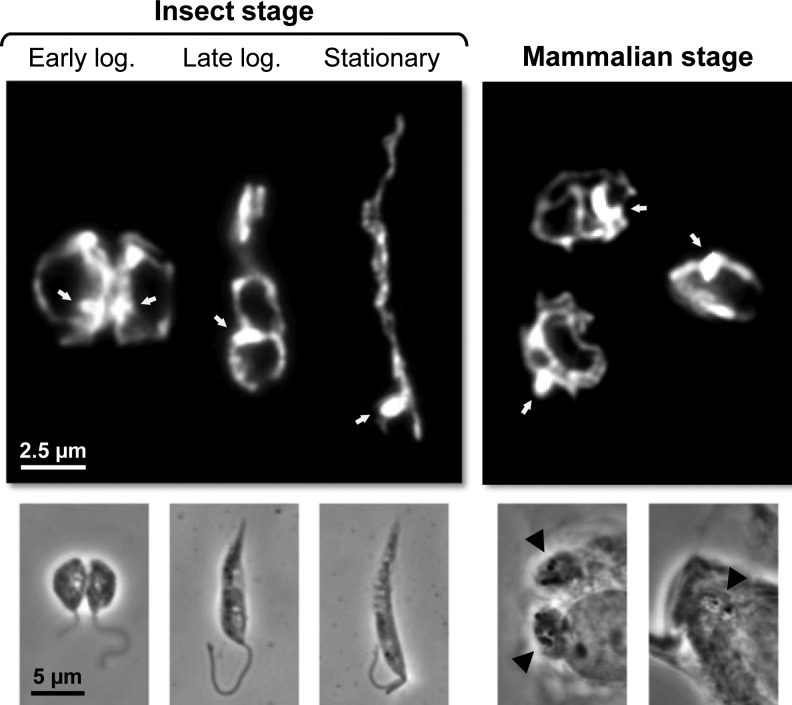
Several pieces of evidence, including recent data on the characterization of the mitochondria protein-import machinery (75), indicate that trypanosomatids are among the earliest diverging eukaryotes to have mitochondria (43). In these organisms, this organelle is a single structure (68) with a tubular form which extends throughout the cell. It contains an outer membrane, a dense matrix, and an inner membrane that can fold into thin and irregularly distributed cristae whose number varies widely (25). In fact, one characteristic of trypanosomatid mitochondria is their plasticity, with the exact morphology, volume, and activity depending on factors such as the cell cycle phase, the stage, and the species, or the nutritional sources available (25). Figure 2 illustrates the alterations in the morphology of Leishmania infantum mitochondria along parasite development. The variability in trypanosomatid mitochondria is even more striking in T. brucei. While in procyclic parasites thriving at the expenses of amino acids such as proline, mitochondria are large and highly branched; in bloodstream forms, which obtain their energy solely from glycolysis, they are small and repressed. Within the mitochondrial matrix and located perpendicular to the axis of the flagellum, lies the kinetoplast, a disk-shaped structure that contains the mitochondrial DNA (the kinetoplast DNA or kDNA) which in trypanosomatids is composed of a dense network of two types of circular molecules, maxicircles and minicircles. The former encode some of the mitochondrial proteins while the second specify unique RNA molecules required for RNA editing, a distinctive process occurring within trypanosomatid mitochondria which converts aberrant RNA sequences into functional messages (7). Despite singularities such as the ones mentioned above, many of the physiologic processes taking place in mitochondria of eukaryotes are also present in trypanosomatids. Still, some of these may exhibit unique features as is the case of the mechanism for cytochrome c (cyt c) biogenesis (3).
FIG. 2.
Mitochondria plasticity along Leishmania development. Immunofluorescence micrographs showing the tubular mitochondrion of Leishmania infantum stained with an antibody against a mitochondrial protein (LimTXNPx, see text) in different life cycle stages of the parasite (upper panels). Dividing promastigotes (insect stage) in the early and late logarithmic phases of growth present a circular and symmetric mitochondrion. A similar description applies to this organelle in the mammalian stage of L. infantum. This contrasts with the elongated mitochondrion of nondividing (stationary) insect stage forms. Also depicted are the phase contrast pictures of the parasites (lower panels). The mitochondrial DNA (kDNA) is indicated by white arrows. Black arrowheads point to intramacrophagic L. infantum amastigotes. (Castro and Tomás, unpublished work).
ROS Formation Within Trypanosomatid Mitochondria
Physiologic generation of ROS is an unquestionable phenomenon in aerobic organisms, the mitochondrion being an important site for its occurrence. Although technical limitations have up to now precluded the accurate determination of the levels generated in vivo, studies with isolated mitochondria estimate that monoelectronic reduction of molecular oxygen (O2) to superoxide anion (O2•−) accounts for up to 0.1% of the total O2 consumed by resting cells (37). In trypanosomatids, production of ROS within mitochondria is also documented. Evidence collected from a number of studies have established that generation of these molecules can be triggered by many drug compounds (14, 38), including some used in clinics as is the case of miltefosine (52), as well as by classic respiratory chain inhibitors (33, 56, 77). An important question, however, is whether ROS formation does occur along the parasites life cycle. In this regard, studies reporting production of mitochondrial ROS in response to physiologic stimuli, such as serum complement (72) and heat shock (4), and linking the formation of these molecules to induction of apoptotic-like death, are particularly important as they suggest that ROS generation is a phenomenon that can occur in vivo and have functional significance for trypanosomatids.
Although there are solid data associating ROS with trypanosomatid mitochondria, the exact site for their production has not been as thoroughly addressed as in other systems. Of relevance, the isolation of the single mitochondrion of trypanosomatids in an intact form is difficult. Such analyses are, thus, usually carried out either using mitochondrial enriched fractions (vesicles) displaying membrane potential or, more frequently, whole parasites selectively permeabilized with digitonin at concentrations that preserve the integrity of the organelle (85).
In most eukaryotes, the respiratory chain is the main site for ROS production within mitochondria. During transference of reducing equivalents along the several intermediates of the chain, some electrons may escape, allowing for the monovalent reduction of molecular oxygen to superoxide anion (O2•−). This radical ion is the primary ROS formed in cells and the precursor for hydrogen peroxide (H2O2) and other species (48, 51). With the possible exception of T. brucei bloodstream forms, the respiratory chain might as well constitute a source of reactive oxygen species to trypanosomatids. In fact, in spite of differences relative to other eukaryotes, the metabolism of all these organisms also entails electron flow along the chain (11, 59, 62, 83).
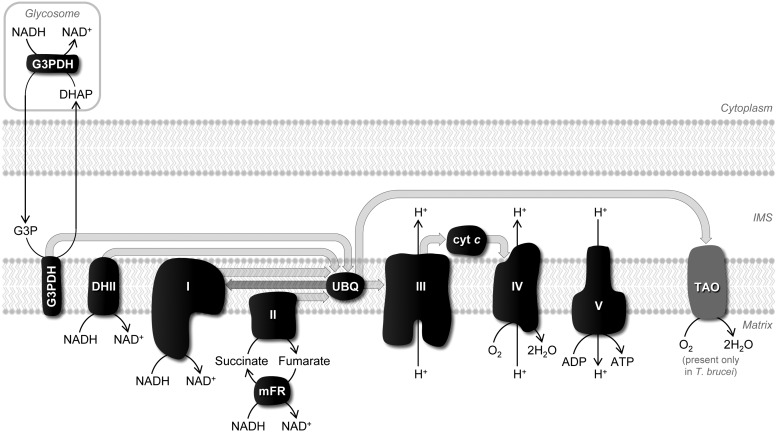
The main features of the respiratory chain of trypanosomatids are depicted in Figure 3. Although there are species and stage differences in the chain, in general terms, electrons from NADH and succinate enter the chain at different points via the mobile carriers ubiquinone (UBQ) and cyt c and are transferred to a terminal oxidase (complex IV). The latter uses this reducing power to produce water from molecular oxygen. Electron transfer through complexes III and IV is coupled to proton translocation promoting the formation of an electrochemical gradient between both sides of the inner mitochondrial membrane that sustains ATP synthesis.
FIG. 3.
Main features of the respiratory chain of trypanosomatids. Reducing equivalents produced during mitochondrial catabolic pathways enter the respiratory chain at complex I, type II NADH-dehydrogenase (DHII), and at complex II, and reduce the pool of ubiquinone (UBQ) in the inner membrane. Glycerol-3-phosphate dehydrogenase (G3PDH), which regenerates glycosomal glycerol-3-phosphate (G3P) back to dihydroxyacetone phosphate (DHAP), also serves as an electron entering point to the respiratory chain. Electrons from UBQ are then successively transported to complex III, to the intermembrane space (IMS) mobile carrier cytochrome c (cyt c) and to complex IV, where they reduce molecular oxygen (O2) to water (H2O). Electron transfer through complexes III and IV is coupled to proton (H+) translocation from the matrix to the IMS. This induces the formation of an electrochemical gradient (ΔΨ) between both sides of the inner mitochondrial membrane, which is used to produce ATP from ADP and inorganic phosphate. In T. brucei bloodstream forms, the respiratory chain is considerably simpler and entails only G3PDH and the trypanosomatid alternative oxidase (TAO). The latter is also present in the T. brucei insect stage but not in Leishmania spp. and T. cruzi. In trypanosomatids, complexes I, II, and III, as well as DHII, are potential sites for superoxide anion generation, as is the matrix enzyme mitochondrial fumarate reductase (mFR). In this scheme, block arrows in light gray represent electron flux along the several components of the scheme. The reverse electron transfer between ubiquinone and complex I is depicted with a dark gray block arrow.
Complex I
In a typical eukaryote, one of the main sites for ROS generation within the respiratory chain is complex I, which releases O2•− on the matrix side (48). In trypanosomatids, this complex shows some singularities, including the absence of proton extrusion activity, but it appears to conserve all subunits containing the redox centers required for UBQ reduction (1, 63, 86). Nevertheless, it is relatively consensual that in all these organisms complex I displays low NADH dehydrogenase activity (1, 13, 77), which could explain why O2•− originating from this complex was not detected neither in T. brucei procyclics (33, 86) nor in T. cruzi epimastigotes (13, 38). Superoxide formation from this locus was, however, reported in Leishmania donovani promastigotes upon stimulation with 100 μM rotenone (56).
Type II NADH dehydrogenase
In addition to complex I, a second inner membrane NADH dehydrogenase (DHII), with flavin as cofactor and also devoid of proton translocation activity, was characterized in T. brucei (35). Owing to the presence of orthologous sequences in the genomes of T. cruzi and Leishmania spp. (Tc00.1047053508717.20 and LinJ.36.5620; http://tritrypdb.org/tritrypdb/, last accessed on August 8, 2012), this molecule should constitute an alternative electron point entry to the respiratory chain of all these organisms. Type II NADH dehydrogenases have also been implicated in ROS production in T. brucei procyclics (33) and, very likely, in T. cruzi epimastigotes (13).
Mitochondrial glycerol 3-phosphate dehydrogenase
Another dehydrogenase present in the inner mitochondrial membrane of all trypanosomatids, but particularly important in bloodstream forms of T. brucei, is glycerol-3-phosphate dehydrogenase (G3PDH) (64). This FAD-dependent enzyme oxidizes glycolysis-derived glycerol-3-phosphate (G3P) forming dihydroxyacetone phosphate (DHAP), as part of a shuttle between the glycosome (the organelle where glycolysis takes place) and the mitochondrion that helps keeping redox balance within the glycosome (Fig. 3). Mitochondrial G3PDH also feeds electrons in the respiratory chain. Although not recognized as a source of ROS in trypanosomatids, analogous enzymes in other organisms generate O2•− to the intermembrane space (24).
Complex II
Apart from NADH, electrons can be supplied to the electron transport chain from succinate to UBQ via complex II, or succinate dehydrogenase. In other eukaryotes, complex II is only seldom referred to as producing O2•− (90), perhaps because structural features of this complex limit accessibility of O2 to FAD (48). ROS production from trypanosomatid complex II was reported in T. cruzi epimastigotes (38, 80) and in L. donovani promastigotes (56) stimulated with the complex II inhibitor thenoyltrifluoroacetone (TTFA).
Reverse electron transfer
Electrons derived from succinate are also often referred to give rise to O2•− through a different mechanism, reverse electron transfer (Fig. 3). In this case, reducing equivalents supplied via complex II to UBQ are transported back to complex I (and in trypanosomatids perhaps to type II NADH dehydrogenase) inducing ROS formation (48, 58). Reverse electron transfer, which is stimulated by a high inner membrane potential and a reduced UBQ pool, was credited as originating O2•− in T. brucei (33) and in T. cruzi (13), but it is debatable whether this mechanism occurs in vivo.
Complex III
Within the respiratory chain of different organisms, complex III (ubiquinol:cytochrome c oxidoreductase) is usually a major source of ROS. Interestingly, Fang and Beattie (33) did not rank this site as a potential local for O2•− generation in T. brucei. On the contrary, in L. donovani promastigotes treated with antimycin (56) or with the primaquine analogue tafenoquine (14), as well as in T. cruzi epimastigotes cultured with Serratia marcescens prodigiosin or stimulated with antimycin (38), O2•− formation was readily triggered at this complex. None of these studies has, however, addressed the submitochondrial compartmentalization of the produced ROS. In other systems, O2•− may be directed to the matrix or to the intermembrane space, depending on which of the two sites within mammalian complex III (designated as Qi and Qo) generates this radical species.
Complex IV and the alternative terminal oxidase of T. brucei
Complex IV, or cytochrome c oxidase, is not regarded as a source of ROS, neither in trypanosomatids nor in other eukaryotes. Nevertheless, a diminished oxidase function of complex IV, either inherent to the parasite or caused by drugs, obviously induces mitochondrial ROS production as any constraint to electron flow favors their escape to O2. A lower activity of complex IV, together with an increased activity of complexes II and III, was referred to as the reason why T. cruzi trypomastigotes generated higher levels of ROS than epimastigotes (41).
When discussing ROS production in trypanosomatid mitochondria, it is important to mention trypanosomatid alternative oxidase (TAO) (Fig. 3). This protein, which is restricted to the inner mitochondrial membrane of both bloodstream and procyclic forms of T. brucei, does not exhibit proton translocation capacity and, therefore, cannot contribute to the proton gradient that drives ATP formation (23), but functions as an electron acceptor. In the parasite mammalian forms, TAO completely replaces complex IV, its function being to oxidize UBQ formed in consequence of G3PDH activity. In the insect stage, TAO coexists with complex IV and, albeit less active than in bloodstream forms (23), it is also functional. In fact, TAO could substitute for complex IV when this was silenced by RNAi (46). Interestingly, it was suggested that, by assisting complex IV in removing excess reducing equivalents and using these to reduce O2 to water, TAO could function in procyclics to decrease mitochondrial O2•− (34).
Fumarate reductase as a potential site for ROS generation
In addition to the classic respiratory chain components, other mitochondrial NADH-dehydrogenases may leak electrons directly to O2 and, in consequence, give rise to O2•− (48). In trypanosomatids one of these is mitochondrial fumarate reductase (mFR), a Krebs cycle enzyme that regenerates succinate from fumarate at the expenses of NADH in what constitutes a peculiarity of these parasites (Fig. 3). Mitochondrial fumarate reductase was demonstrated to be a potential site for O2•− formation in T. brucei and T. cruzi insect stages (13, 26, 33, 84).
ROS Elimination in Trypanosomatid Mitochondria
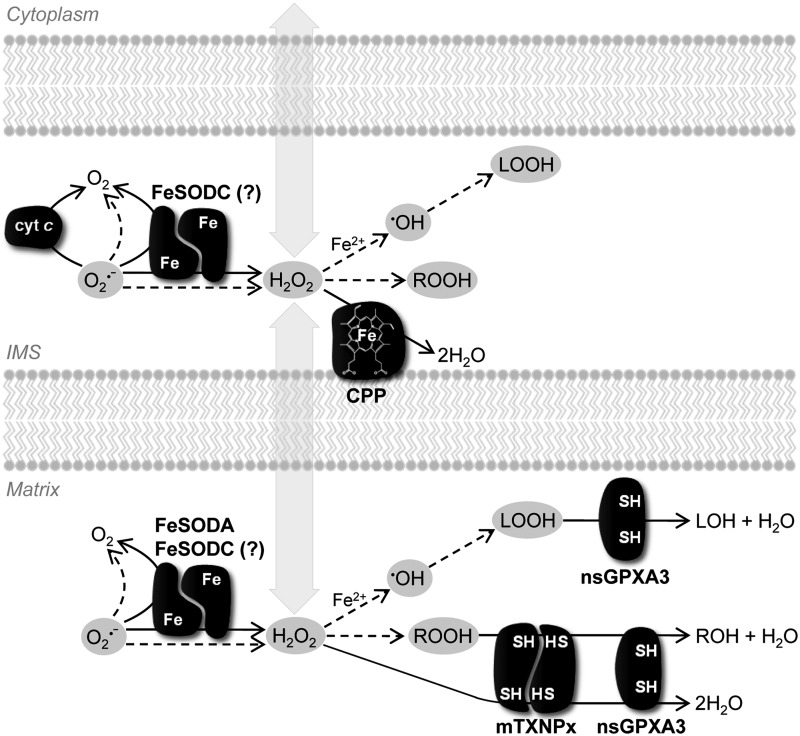
Superoxide anion formed in mitochondria may give rise to other ROS (Fig. 4). Among these, H2O2 may cross biological membranes (8) and exert its effects in other subcellular compartments. Apart from participating in physiologic processes (e.g., by regulating signaling pathways) (10), H2O2 may have deleterious consequences on cells owing to its capacity to react with biological molecules as well as to originate the highly reactive hydroxyl radical (•OH). The fine regulation of ROS levels in mitochondria is thus required, so that the cytotoxic impact of these molecules is circumvented without compromising their physiologic functions. Enzymes with ROS-eliminating activity as well as nonenzymatic mechanisms are implicated in such control. Next, we will review the available information on the antioxidant enzymes present in trypanosomatid mitochondria (Fig. 5).
FIG. 4.
Routes for formation and elimination of ROS in trypanosomatid mitochondria. This scheme shows the main routes for ROS generation from superoxide anion, as well as the antioxidant enzymes described to be present in trypanosomatid mitochondria. Superoxide anion (O2•−), derived from the single-electron reduction of molecular oxygen (O2) either in the matrix or in the intermembrane space (IMS), is the precursor of most ROS. It may be eliminated via cytochrome c (cyt c)-driven oxidation to O2 in the IMS. Alternatively, it may be dismutated to O2 and hydrogen peroxide (H2O2), either spontaneously or catalytically by FeSODA and FeSODC [the uncertain localization of the latter molecule, either to the IMS or to the matrix (see main text), is indicated by a question mark]. Hydrogen peroxide is the oxidizing substrate of CCP in the IMS, as well as of mTXNPx and nsGPXA3 in the matrix. If not eliminated by these enzymes, H2O2 can diffuse across membranes, participate in signaling pathways, or react with biological molecules to generate organic hydroperoxides (ROOH). Importantly, H2O2 can originate hydroxyl radical (•OH) via oxidation of a transition metal ion, such as Fe2+ (the “Fenton reaction”). The hydroxyl radical is a powerful oxidant, reacting directly with nucleic acids and proteins, and initiating lipid peroxidation (LOOH). Some of the products resulting from hydrogen peroxide- and hydroxyl radical-driven reactions, namely ROOH and LOOH, can be reduced to the corresponding alcohols (ROH and LOH) by mTXNPx and/or nsGPXA3. In this scheme, ROS are highlighted in light gray ovals and the various ROS-eliminating enzymes are represented in dark gray shapes. Noncatalyzed and catalyzed reactions are distinguished by dashed and filled arrows, respectively. Trypanosomatid FeSODs and mTXNPx are depicted as homodimeric enzymes.
FIG. 5.
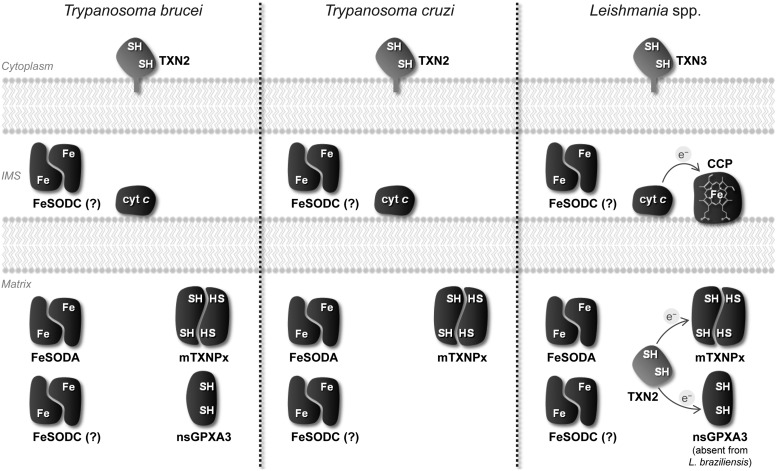
Comparison of the mitochondrial antioxidant enzymes in T. brucei, T. cruzi, and Leishmania spp. This diagram shows the various mitochondrial ROS-eliminating enzymes in trypanosomatids. Depicted are the enzymes FeSODA, mTXNPx, and nsGPXA3, most likely located in the matrix. The latter enzyme has no obvious counterpart in T. cruzi and L. braziliensis. Also in the mitochondrial matrix is the TXN2 molecule of Leishmania spp., which supplies reducing equivalents (represented with an “eē”) to mTXNPx and nsGPXA3. All trypanosomatids harbor a second TXN molecule in their mitochondrion, TXN2 in T. brucei and T. cruzi, and TXN3 in Leishmania spp. This protein is anchored to the outer mitochondrial membrane by a hydrophobic C-tail, with the redox active site facing the cytosol. The intermembrane space (IMS) is equipped with cytochrome c (cyt c) and presumably with FeSODC (albeit this enzyme may alternatively be located in the matrix). Leishmania spp., but not the other trypanosomatids, harbor one CCP enzyme in the IMS, which is reduced by cyt c.
Superoxide dismutases
Elimination of O2•− is an adequate means to regulate the formation of other ROS. Even though this may occur by the spontaneous dismutation of superoxide to H2O2 and O2, this reaction is accelerated by the activity of superoxide dismutases (SODs) (55). These enzymes dismutate O2•− following a mechanism that involves a metal (M) co-factor, as detailed next:
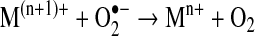 |
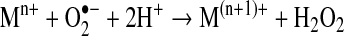 |
Trypanosomatid mitochondria are equipped with two SODs, FeSODA and FeSODC (30, 89) (Fig. 4). Noticeably, these two enzymes utilize iron as metal co-factor and not manganese as mitochondrial SODs of other eukaryotes. This dependence on iron is reminiscent of SODs of prokaryotes and plant chloroplasts. The submitochondrial compartmentalization of FeSODA and FeSODC has never been experimentally addressed, but, at least in the case of the former enzyme, bioinformatic analysis supports its residence in the matrix (9). As for FeSODC, its unusually large N-terminal signal peptide was suggested by Dufernez et al. to target the enzyme to the intermembrane space (30) and by Bodył et al. (9) to the matrix, its final destination remaining doubtful. As a note, one alternative means to remove O2•− in this specific compartment is via its cyt c-driven oxidation to O2 (Fig. 4), in analogy to what is described for other systems (70). Additionally, it is conceivable that, as reported for other organisms, O2•− moves through anion channels into the cytosol (44) where it can be eliminated by local antioxidants.
The role of both FeSODA and FeSODC as antioxidants has been evidenced by forward and reverse genetics. Illustrating this, overexpression of FeSODA was reported to protect Leishmania from the free radical generating agents paraquat, nitroprusside (67), and antimycin (39), whereas downregulation of FeSODC by antisense RNA rendered Leishmania tropica promastigotes more sensitive to menadione (40), a known O2•− inducer. Likewise, knocking down of FeSODA by RNAi had a negative impact on the resistance of T. brucei bloodstream form parasites to paraquat, a phenotype that was not reproduced by FeSODC-deficient parasites (89). Even though FeSODA and FeSODC are apparently active as antioxidants in the parasite context, neither enzyme is crucial for survival of bloodstream T. brucei parasites (89). This fact may result from a mutual compensation of function or it may reflect an insufficient generation of O2•− by the rudimentary mitochondrion of T. brucei at this life stage. In Leishmania the impact of mitochondrial SODs on parasite survival appears more prominent, as lack of FeSODC resulted in reduced survival of L. donovani amastigotes in mouse macrophages (40). In the case of T. cruzi, the report that FeSODA expression is upregulated in infective metacyclic trypomastigotes relative to noninfective epimastigotes (6) suggests that this enzyme may also play a role in parasite adaptation to the mammalian host. This observation may, nevertheless, apply only to some parasite strains, as it was reported that FeSODA expression may vary with the T. cruzi lineage (69). One final note to mention that the antioxidant activity of FeSODA may also account for its involvement in the regulation of apoptotic-like death, as suggested for both T. cruzi (72) and L. donovani (39).
Cytochrome c peroxidase
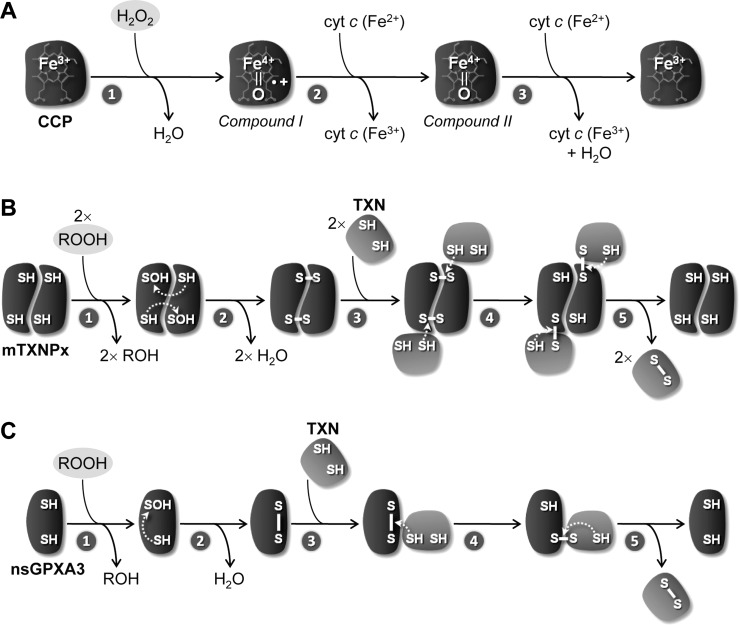
Within trypanosomatid mitochondria H2O2, the product of O2•− dismutation, can be removed by different peroxidases. One of these is cytochrome c peroxidase (CCP), a heme-containing enzyme that is present in Leishmania spp. Originally described in Leishmania major (LmCCP) as an ascorbate-dependent peroxidase (2), this enzyme is now known to be preferentially reduced by ferrous cyt c (47) following the mechanism depicted in Figure 6A. LmCCP is localized in the inner mitochondrial membrane with its catalytic domain facing the intermembrane space (28). The enzyme plays an antioxidant role (28, 29, 66), but it is redundant throughout the L. major life cycle (66). This observation is not entirely surprising if one takes into consideration that LmCCP has no obvious representative in T. cruzi and T. brucei (Fig. 5). A specific review on LmCCP is presented in this Forum Issue by Adak and Pal.
FIG. 6.
Catalytic mechanism of cytochrome c peroxidase and of thiol peroxidases. (A) 1. In the first step of cytochrome c peroxidase (CCP) catalysis, the enzyme reacts with H2O2 in a two-electron redox reaction that leads to the heterolytic cleavage of the hydroperoxide and the release of a molecule of water. In the resulting oxidized enzyme (Compound I), the second H2O2-derived oxygen atom oxidizes the heme iron to a Fe4+=O oxyferryl group and a nearby tryptophan residue to a cationic radical (•+). 2. This radical is subsequently reduced by one molecule of ferrous cyt c [cyt c (Fe2+)] to generate Compound II, which maintains the Fe4+=O center. 3. The enzyme is reduced back to the resting ferric state by a second one-electron transfer from another cyt c (Fe2+) molecule, with release of water. (B) and (C) The thiol peroxidases mTXNPx and nsGPXA3 share the same steps of catalysis, with the difference that the former reacts as a dimer and the latter as a monomer. 1. First, the proximal Cys of the peroxidase reacts with ROOH to yield ROH and sulfenic acid (-SOH). 2. A disulfide bond is then formed inter- (in the case of mTXNPx) or intramolecularly (for nsGPXA3) between the oxidized Cys and a distal thiol group, with concomitant release of water. 3. The disulfide is subsequently attacked by a Cys thiol group from tryparedoxin (TXN). 4. A catalytic intermediate is generated, in which the proximal Cys of the peroxidase is restored to its reduced (-SH) state and the distal Cys is covalently bound to TXN via an S-S bridge. 5. The reaction cycle is completed by a thiol-disulfide exchange reaction, in which the mixed disulfide is resolved by the second Cys residue of TXN, thus releasing the fully reduced peroxidase and the reducing substrate in its oxidized (S-S) form.
Thiol peroxidases
The other hydroperoxide-reducing enzymes operating within trypanosomatid mitochondria are two thiol peroxidases, namely a 2-Cys-peroxiredoxin enzyme, designated mTXNPx (16, 82, 87) [or, according to the recent nomenclature proposed by Gretes et al. (42), Prx1m], and a nonselenium glutathione peroxidase, referred to as nsGPXA3 (45, 79). The activity of these molecules does not require any cofactor or prosthetic group and, instead, depends on the reactivity of two redox active cysteines. The mechanisms of reaction of these two classes of enzymes, originally dissected by Rhee (22), Poole (31), and Maiorino (53), are depicted in Figure 6B and 6C.
Both mTXNPx and nsGPXA3 are assumed to be present in the mitochondrial matrix, where they can exert their activity as H2O2-reducing enzymes (16, 74, 78) (Fig. 4). In addition to H2O2, these two enzymes accept small organic hydroperoxides (18, 27, 78) and, in the case of nsGPXA3, lipid hydroperoxides (27, 78) as oxidizing substrates.
Consistent with the in vitro activity of mTXNPx as a peroxidase, its ectopic overexpression was shown to confer parasites resistance towards H2O2 of exogenous origins (16, 49, 87). Based on these observations, the main physiologic function of mTXNPx was for a long time regarded to be that of an antioxidant. This concept was, nevertheless, challenged by a recent report demonstrating that the peroxidase function of mTXNPx is not crucial, even though the protein itself is essential during the mammalian stage of L. infantum (20). Instead, it is possibly the chaperone-like activity of mTXNPx that is required for amastigote replication (20). In T. cruzi, mTXNPx is also expected to be relevant for parasite survival in the vertebrate host, as suggested by the observations that this enzyme is upregulated in virulent T. cruzi strains (71) or when these parasites differentiate into metacyclic forms (6). Moreover, mTXNPx overexpression was found to enhance T. cruzi survival in mammalian cells (73). For the T. cruzi mTXNPx molecule, however, the actual contribution of its peroxidase activity for parasite survival remains to be investigated, following on the abovementioned report that its L. infantum counterpart displays an alternative function (20). In what concerns the mitochondrial nsGPXA3, functional studies in T. brucei revealed that this enzyme is not vital, its depletion having resulted in cardiolipin peroxidation and in a transient retardation of growth (27). That finding agrees with the fact that other trypanosomatids, namely T. cruzi and Leishmania braziliensis, can thrive in the absence of a nsGPXA3 orthologue (Fig. 5). Altogether, these observations suggest that when eliminated individually the mitochondrial thiol peroxidases, or more precisely their peroxidase activity, are not vital to trypanosomatids. One possible explanation for this redundancy could be that one molecule might overcome the absence of the other, even though they have different preferences for hydroperoxides. A definitive conclusion about the relevance of both mitochondrial thiol peroxidases as antioxidant devices would, hence, require their simultaneous down regulation. Another unsolved issue regarding mitochondrial thiol peroxidases is their physiologic source of reducing equivalents, as explained next.
Thiol peroxidases are typically reduced by CxxC-containing oxidoreductases of the thioredoxin family (which includes thioredoxins and glutaredoxins) or by proteins possessing thioredoxin-like active sites (Fig. 6B and 6C). Trypanosomatids harbor a specific thioredoxin-like subfamily of proteins, known as tryparedoxins (TXNs) (60), which are the preferred reducing substrates for thiol peroxidases in these organisms. Tryparedoxins are nonenzymatically reduced by trypanothione [T(SH)2] (32), a conjugate of glutathione and spermidine that largely replaces glutathione functions in trypanosomatids and that is itself maintained in the reduced state by the flavoenzyme trypanothione reductase (TR) at the expenses of NADPH. Whereas in biochemical assays, both mTXNPx and nsGPXA3 are reduced by the TR/T(SH)2/TXN system (18, 74, 78), it is not clear how this reaction proceeds in the parasite context. This doubt stems from three different reasons. First, the evidence for the presence of TR in mitochondria is conflicting. In an immunolocalization study in T. cruzi (45), TR was reported to partially localize to the mitochondrion (57), but this observation was not corroborated by subsequent studies (15, 78, 81, 88) based on subcellular fractionations of T. brucei, T. cruzi, and L. infantum (13, 58, 61, 67). Second, it is not known whether T(SH)2 is present in mitochondria. Since trypanothione synthetase appears to be restricted to the cytosol (65), the thiol would have to be imported into the organelle by means of carriers either specific or not. Up to now, no such transporters have been identified. Third, the requirement for a mitochondrial TXN is also questionable as trypanosomatids can thrive without a TXN molecule in this cell compartment (17). These organisms do possess a mitochondrial TXN-like enzyme (TXN3 in Leishmania spp. or TXN2 in T. cruzi and T. brucei), but this is a mitochondrial outer membrane protein that has no possibility to interact directly with, hence reduce, mTXNPx and nsGPXA3 (Fig. 5). Leishmania spp., but not T. cruzi and T. brucei, harbor an additional mitochondrial TXN molecule (the Leishmania TXN2) (19) that is, nevertheless, redundant (17). In this context, it is worth mentioning that the nonessentiality of a mitochondrial TXN also impacts on other proposed TXN-dependent mechanisms taking place in this organelle, namely the reduction of the universal minicircle sequence binding protein (UMSBP) that is required for kDNA replication (61).
In the absence of a mitochondrial TXN, reduction of thiol peroxidases might be carried out by other oxidoreductases containing both a CxxC motif and a thioredoxin domain. Among these, thioredoxins and glutaredoxins are unlikely to exhibit such activity. The latter, despite having representatives in the mitochondrion of these parasites (21, 36), are unable to replace TXN as reductants of thiol peroxidases (21, 54). As for thioredoxin, although the T. brucei enzyme was shown to reduce TXNPx (79) and nsGPXA3 (45) in vitro, its presence in the mitochondrion was never demonstrated. In what concerns other putative dithiol-containing oxidoreductases predicted in the genome of trypanosomatids, it is difficult to infer about their role as reductants for thiol peroxidases without experimental evidence.
Nonenzymatic scavengers of hydroperoxides in trypanosomatid mitochondria
In trypanosomatid mitochondria hydroperoxides may also be eliminated nonenzymatically. Since T(SH)2 is capable of scavenging H2O2 directly (12, 45), this thiol comes out as an attractive candidate to perform such a role, providing its presence in the organelle is ascertained. Apart from trypanothione, other low molecular mass thiols [e.g., glutathione, ovothiol (5), or free cysteine] as well as thiols exposed on protein surfaces (76) and other antioxidants (e.g., ascorbate) might contribute to mitochondrial antioxidant function. Determination of the concentration of trypanothione and of other thiols in the mitochondria of the several trypanosomatids will help elucidating the importance of nonenzymatic mechanisms on hydroperoxide removal in this organelle.
Conclusion
In spite of species and life cycle stage differences, mitochondria of trypanosomatids have the potential to generate ROS, either in the respiratory chain or in other sites. These organelles are equipped with several antioxidant enzymes, two of which dismutate O2•− (FeSODA and FeSODC) and three that reduce H2O2 and other hydroperoxides (CCP, mTXNPx and nsGPXA3). Interestingly, all five enzymes are only preserved in Leishmania spp., with T. brucei and T. cruzi missing one or two of these molecules (Fig. 5). This fact, added to the findings that the antioxidant activity of these enzymes is not crucial for parasite survival, suggests that their functions may overlap and/or may be efficiently replaced by nonenzymatic scavengers of ROS. The redundant role of mTXNPx and nsGPXA3 as peroxidases is in agreement with the nonessential character of their preferred reducing agent (TXN) within mitochondria. In the future, the simultaneous depletion of several redundant antioxidant enzymes will certainly shed light on the relative importance of catalytic elimination of ROS in trypanosomatids.
Abbreviations Used
- CCP
cytochrome c peroxidase
- Cys
cysteine
- cyt c
cytochrome c
- DHAP
dihydroxyacetone phosphate
- DHII
type II NADH dehydrogenase
- FeSOD
iron superoxide dismutase
- FR
fumarate reductase
- G3P
glycerol-3-phosphate
- G3PDH
glycerol-3-phosphate dehydrogenase
- IMS
intermembrane space
- kDNA
kinetoplast DNA
- LOOH
lipid hydroperoxides
- mTXNPx
mitochondrial tryparedoxin peroxidase
- nsGPXA3
mitochondrial nonselenium glutathione peroxidase
- RNAi
RNA interference
- ROOH
small organic hydroperoxides
- ROS
reactive oxygen species
- TAO
trypanosomatid alternative oxidase
- TR
trypanothione reductase
- T(SH)2
trypanothione (reduced form)
- TXN
tryparedoxin
- UBQ
ubiquinone
Acknowledgments
The authors acknowledge Fundação para a Ciência e a Tecnologia, Portugal (grants SFRH/BPD/80836/2011, PTDC/CVT/70275/2006, PTDC/BIA-MIC/100910/2008 and PTDC/CVT/100090/2008) and Fundação Calouste Gulbenkian, Portugal (grant P-105335) for continuous financial support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Acestor N. Zíková A. Dalley RA. Anupama A. Panigrahi AK. Stuart KD. Trypanosoma brucei mitochondrial respiratome: Composition and organization in procyclic form. Mol Cell Proteomics. 2011;10:M110.006908. doi: 10.1074/mcp.M110.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adak S. Datta AK. Leishmania major encodes an unusual peroxidase that is a close homologue of plant ascorbate peroxidase: A novel role of the transmembrane domain. Biochem J. 2005;390:465–474. doi: 10.1042/BJ20050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JW. Jackson AP. Rigden DJ. Willis AC. Ferguson SJ. Ginger ML. Order within a mosaic distribution of mitochondrial c-type cytochrome biogenesis systems? FEBS J. 2008;275:2385–2402. doi: 10.1111/j.1742-4658.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 4.Alzate JF. Arias AA. Moreno-Mateos D. Alvarez-Barrientos A. Jiménez-Ruiz A. Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol Biochem Parasitol. 2007;152:192–202. doi: 10.1016/j.molbiopara.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Ariyanayagam MR. Fairlamb AH. Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol Biochem Parasitol. 2001;115:189–198. doi: 10.1016/s0166-6851(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 6.Atwood JA., III Weatherly DB. Minning TA. Bundy B. Cavola C. Opperdoes FR. Orlando R. Tarleton RL. The Trypanosoma cruzi proteome. Science. 2005;309:473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- 7.Benne R. Van den Burg J. Brakenhoff JP. Sloof P. Van Boom JH. Tromp MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- 8.Bienert GP. Schjoerring JK. Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Bodył A. Mackiewicz P. Were class C iron-containing superoxide dismutases of trypanosomatid parasites initially imported into a complex plastid? A hypothesis based on analyses of their N-terminal targeting signals. Parasitology. 2008;135:1101–1110. doi: 10.1017/S0031182008004642. [DOI] [PubMed] [Google Scholar]
- 10.Brigelius-Flohé R. Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bringaud F. Barrett MP. Zilberstein D. Multiple roles of proline transport and metabolism in trypanosomatids. Front Biosci. 2012;17:349–374. doi: 10.2741/3931. [DOI] [PubMed] [Google Scholar]
- 12.Carnieri EG. Moreno SN. Docampo R. Trypanothione-dependent peroxide metabolism in Trypanosoma cruzi different stages. Mol Biochem Parasitol. 1993;61:79–86. doi: 10.1016/0166-6851(93)90160-y. [DOI] [PubMed] [Google Scholar]
- 13.Carranza JC. Kowaltowski AJ. Mendonça MA. de Oliveira TC. Gadelha FR. Zingales B. Mitochondrial bioenergetics and redox state are unaltered in Trypanosoma cruzi isolates with compromised mitochondrial complex I subunit genes. J Bioenerg Biomembr. 2009;41:299–308. doi: 10.1007/s10863-009-9228-4. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho L. Luque-Ortega JR. Manzano JI. Castanys S. Rivas L. Gamarro F. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother. 2010;54:5344–5351. doi: 10.1128/AAC.00790-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro H. Romao S. Gadelha FR. Tomás AM. Leishmania infantum: Provision of reducing equivalents to the mitochondrial tryparedoxin/tryparedoxin peroxidase system. Exp Parasitol. 2008;120:421–423. doi: 10.1016/j.exppara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Castro H. Sousa C. Santos M. Cordeiro-da-Silva A. Flohé L. Tomás AM. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic Biol Med. 2002;33:1552–1562. doi: 10.1016/s0891-5849(02)01089-4. [DOI] [PubMed] [Google Scholar]
- 17.Castro H. Romao S. Carvalho S. Teixeira F. Sousa C. Tomás AM. Mitochondrial redox metabolism in trypanosomatids is independent of tryparedoxin activity. PLoS One. 2010;5:e12607. doi: 10.1371/journal.pone.0012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro H. Budde H. Flohé L. Hofmann B. Lünsdorf H. Wissing J. Tomás AM. Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic Biol Med. 2002;33:1563–1574. doi: 10.1016/s0891-5849(02)01088-2. [DOI] [PubMed] [Google Scholar]
- 19.Castro H. Sousa C. Novais M. Santos M. Budde H. Cordeiro-da-Silva A. Flohé L. Tomás AM. Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol Biochem Parasitol. 2004;136:137–147. doi: 10.1016/j.molbiopara.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Castro H. Teixeira F. Romao S. Santos M. Cruz T. Flórido M. Appelberg R. Oliveira P. Ferreira-da-Silva F. Tomás AM. Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: Insight into its novel chaperone activity. PLoS Pathog. 2011;7:e1002325. doi: 10.1371/journal.ppat.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceylan S. Seidel V. Ziebart N. Berndt C. Dirdjaja N. Krauth-Siegel RL. The dithiol glutaredoxins of african trypanosomes have distinct roles and are closely linked to the unique trypanothione metabolism. J Biol Chem. 2010;285:35224–35237. doi: 10.1074/jbc.M110.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae HZ. Chung SJ. Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 23.Chaudhuri M. Ott RD. Hill GC. Trypanosome alternative oxidase: From molecule to function. Trends Parasitol. 2006;22:484–491. doi: 10.1016/j.pt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury SK. Gemin A. Singh G. High activity of mitochondrial glycerophosphate dehydrogenase and glycerophosphate-dependent ROS production in prostate cancer cell lines. Biochem Biophys Res Commun. 2005;333:1139–1145. doi: 10.1016/j.bbrc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 25.de Souza W. Attias M. Rodrigues JC. Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida) Int J Biochem Cell Biol. 2009;41:2069–2080. doi: 10.1016/j.biocel.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Denicola-Seoane A. Rubbo H. Prodanov E. Turrens JF. Succinate-dependent metabolism in Trypanosoma cruzi epimastigotes. Mol Biochem Parasitol. 1992;54:43–50. doi: 10.1016/0166-6851(92)90093-y. [DOI] [PubMed] [Google Scholar]
- 27.Diechtierow M. Krauth-Siegel RL. A tryparedoxin-dependent peroxidase protects African trypanosomes from membrane damage. Free Radic Biol Med. 2011;51:856–868. doi: 10.1016/j.freeradbiomed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Dolai S. Yadav RK. Pal S. Adak S. Leishmania major ascorbate peroxidase overexpression protects cells against reactive oxygen species-mediated cardiolipin oxidation. Free Radic Biol Med. 2008;45:1520–1529. doi: 10.1016/j.freeradbiomed.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Dolai S. Yadav RK. Pal S. Adak S. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot Cell. 2009;8:1721–1731. doi: 10.1128/EC.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufernez F. Yernaux C. Gerbod D. Noël C. Chauvenet M. Wintjens R. Edgcomb VP. Capron M. Opperdoes FR. Viscogliosi E. The presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: Characterization, subcellular localization, and phylogenetic origin in Trypanosoma brucei. Free Radic Biol Med. 2006;40:210–225. doi: 10.1016/j.freeradbiomed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Ellis HR. Poole LB. Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry. 1997;36:13349–13356. doi: 10.1021/bi9713658. [DOI] [PubMed] [Google Scholar]
- 32.Fairlamb AH. Blackburn P. Ulrich P. Chait BT. Cerami A. Trypanothione: A novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985;227:1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- 33.Fang J. Beattie DS. Rotenone-insensitive NADH dehydrogenase is a potential source of superoxide in procyclic Trypanosoma brucei mitochondria. Mol Biochem Parasitol. 2002;123:135–142. doi: 10.1016/s0166-6851(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 34.Fang J. Beattie DS. Alternative oxidase present in procyclic Trypanosoma brucei may act to lower the mitochondrial production of superoxide. Arch Biochem Biophys. 2003;414:294–302. doi: 10.1016/s0003-9861(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 35.Fang J. Beattie DS. Identification of a gene encoding a 54 kDa alternative NADH dehydrogenase in Trypanosoma brucei. Mol Biochem Parasitol. 2003;127:73–77. doi: 10.1016/s0166-6851(02)00305-5. [DOI] [PubMed] [Google Scholar]
- 36.Filser M. Comini MA. Molina-Navarro MM. Dirdjaja N. Herrero E. Krauth-Siegel RL. Cloning, functional analysis, and mitochondrial localization of Trypanosoma brucei monothiol glutaredoxin-1. Biol Chem. 2008;389:21–32. doi: 10.1515/BC.2007.147. [DOI] [PubMed] [Google Scholar]
- 37.Fridovich I. Mitochondria: Are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 38.Genes C. Baquero E. Echeverri F. Maya JD. Triana O. Mitochondrial dysfunction in Trypanosoma cruzi: the role of Serratia marcescens prodigiosin in the alternative treatment of Chagas disease. Parasit Vectors. 2011;4:66. doi: 10.1186/1756-3305-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Getachew F. Gedamu L. Leishmania donovani mitochondrial iron superoxide dismutase A is released into the cytosol during miltefosine induced programmed cell death. Mol Biochem Parasitol. 2012;183:42–51. doi: 10.1016/j.molbiopara.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh S. Goswami S. Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem J. 2003;369:447–452. doi: 10.1042/BJ20021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonçalves RL. Barreto RF. Polycarpo CR. Gadelha FR. Castro SL. Oliveira MF. A comparative assessment of mitochondrial function in epimastigotes and bloodstream trypomastigotes of Trypanosoma cruzi. J Bioenerg Biomembr. 2011;43:651–661. doi: 10.1007/s10863-011-9398-8. [DOI] [PubMed] [Google Scholar]
- 42.Gretes MC. Poole LB. Karplus PA. Peroxiredoxins in parasites. Antioxid Redox Signal. 2011;17:608–633. doi: 10.1089/ars.2011.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampl V. Hug L. Leigh JW. Dacks JB. Lang BF. Simpson AG. Roger AJ. Phylogenomic analyses support the monophyly of Excavata and resolve relationships among eukaryotic “supergroups”. Proc Natl Acad Sci USA. 2009;106:3859–3864. doi: 10.1073/pnas.0807880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han D. Antunes F. Canali R. Rettori D. Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 45.Hillebrand H. Schmidt A. Krauth-Siegel RL. A second class of peroxidases linked to the trypanothione metabolism. J Biol Chem. 2003;278:6809–6815. doi: 10.1074/jbc.M210392200. [DOI] [PubMed] [Google Scholar]
- 46.Horváth A. Horáková E. Dunajcáková P. Verner Z. Pravdová E. Slapetová I. Cuninková L. Lukes J. Downregulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei. Mol Microbiol. 2005;58:116–130. doi: 10.1111/j.1365-2958.2005.04813.x. [DOI] [PubMed] [Google Scholar]
- 47.Jasion VS. Poulos TL. Leishmania major peroxidase is a cytochrome c peroxidase. Biochemistry. 2012;51:2453–2460. doi: 10.1021/bi300169x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowaltowski AJ. de Souza-Pinto NC. Castilho RF. Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Lin YC. Hsu JY. Chiang SC. Lee ST. Distinct overexpression of cytosolic and mitochondrial tryparedoxin peroxidases results in preferential detoxification of different oxidants in arsenite-resistant Leishmania amazonensis with and without DNA amplification. Mol Biochem Parasitol. 2005;142:66–75. doi: 10.1016/j.molbiopara.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Loschen G. Flohé L. Chance B. Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 51.Loschen G. Azzi A. Richter C. Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 52.Luque-Ortega JR. Rivas L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2007;51:1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiorino M. Ursini F. Bosello V. Toppo S. Tosatto SC. Mauri P. Becker K. Roveri A. Bulato C. Benazzi L, et al. The thioredoxin specificity of Drosophila GPx: A paradigm for a peroxiredoxin-like mechanism of many glutathione peroxidases. J Mol Biol. 2007;365:1033–1046. doi: 10.1016/j.jmb.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 54.Marquez VE. Arias DG. Piattoni CV. Robello C. Iglesias AA. Guerrero SA. Cloning, expression, and characterization of a dithiol glutaredoxin from Trypanosoma cruzi. Antioxid Redox Signal. 2010;12:787–792. doi: 10.1089/ars.2009.2907. [DOI] [PubMed] [Google Scholar]
- 55.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 56.Mehta A. Shaha C. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: Complex II inhibition results in increased pentamidine cytotoxicity. J Biol Chem. 2004;279:11798–11813. doi: 10.1074/jbc.M309341200. [DOI] [PubMed] [Google Scholar]
- 57.Meziane-Cherif D. Aumercier M. Kora I. Sergheraert C. Tartar A. Dubremetz JF. Ouaissi MA. Trypanosoma cruzi: Immunolocalization of trypanothione reductase. Exp Parasitol. 1994;79:536–541. doi: 10.1006/expr.1994.1114. [DOI] [PubMed] [Google Scholar]
- 58.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naderer T. Ellis MA. Sernee MF. de Souza DP. Curtis J. Handman E. McConville MJ. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc Natl Acad Sci USA. 2006;103:5502–5507. doi: 10.1073/pnas.0509196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nogoceke E. Gommel DU. Kiess M. Kalisz HM. Flohé L. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem. 1997;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 61.Onn I. Milman-Shtepel N. Shlomai J. Redox potential regulates binding of universal minicircle sequence binding protein at the kinetoplast DNA replication origin. Eukaryot Cell. 2004;3:277–287. doi: 10.1128/EC.3.2.277-287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Opperdoes FR. Coombs GH. Metabolism of Leishmania: Proven and predicted. Trends Parasitol. 2007;23:149–158. doi: 10.1016/j.pt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Opperdoes FR. Michels PA. Complex I of Trypanosomatidae: Does it exist? Trends Parasitol. 2008;24:310–317. doi: 10.1016/j.pt.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Opperdoes FR. Borst P. Bakker S. Leene W. Localization of glycerol-3-phosphate oxidase in the mitochondrion and particulate NAD+-linked glycerol-3-phosphate dehydrogenase in the microbodies of the bloodstream form to Trypanosoma brucei. Eur J Biochem. 1977;76:29–39. doi: 10.1111/j.1432-1033.1977.tb11567.x. [DOI] [PubMed] [Google Scholar]
- 65.Oza SL. Shaw MP. Wyllie S. Fairlamb AH. Trypanothione biosynthesis in Leishmania major. Mol Biochem Parasitol. 2005;139:107–116. doi: 10.1016/j.molbiopara.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Pal S. Dolai S. Yadav RK. Adak S. Ascorbate peroxidase from Leishmania major controls the virulence of infective stage of promastigotes by regulating oxidative stress. PLoS One. 2010;5:e11271. doi: 10.1371/journal.pone.0011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paramchuk WJ. Ismail SO. Bhatia A. Gedamu L. Cloning, characterization and overexpression of two iron superoxide dismutase cDNAs from Leishmania chagasi: Role in pathogenesis. Mol Biochem Parasitol. 1997;90:203–221. doi: 10.1016/s0166-6851(97)00141-2. [DOI] [PubMed] [Google Scholar]
- 68.Paulin JJ. The chondriome of selected trypanosomatids. A three-dimensional study based on serial thick sections and high voltage electron microscopy. J Cell Biol. 1975;66:404–413. doi: 10.1083/jcb.66.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peloso EF. Gonçalves CC. Silva TM. Ribeiro LH. Piñeyro MD. Robello C. Gadelha FR. Tryparedoxin peroxidases and superoxide dismutases expression as well as ROS release are related to Trypanosoma cruzi epimastigotes growth phases. Arch Biochem Biophys. 2012;520:117–122. doi: 10.1016/j.abb.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 70.Pereverzev MO. Vygodina TV. Konstantinov AA. Skulachev VP. Cytochrome c, an ideal antioxidant. Biochem Soc Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- 71.Piacenza L. Zago MP. Peluffo G. Alvarez MN. Basombrio MA. Radi R. Enzymes of the antioxidant network as novel determiners of Trypanosoma cruzi virulence. Int J Parasitol. 2009;39:1455–1464. doi: 10.1016/j.ijpara.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piacenza L. Irigoín F. Alvarez MN. Peluffo G. Taylor MC. Kelly JM. Wilkinson SR. Radi R. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: Cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J. 2007;403:323–334. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piñeyro MD. Parodi-Talice A. Arcari T. Robello C. Peroxiredoxins from Trypanosoma cruzi: Virulence factors and drug targets for treatment of Chagas disease? Gene. 2008;408:45–50. doi: 10.1016/j.gene.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Piñeyro MD. Arcari T. Robello C. Radi R. Trujillo M. Tryparedoxin peroxidases from Trypanosoma cruzi: high efficiency in the catalytic elimination of hydrogen peroxide and peroxynitrite. Arch Biochem Biophys. 2011;507:287–295. doi: 10.1016/j.abb.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Pusnik M. Schmidt O. Perry AJ. Oeljeklaus S. Niemann M. Warscheid B. Lithgow T. Meisinger C. Schneider A. Mitochondrial preprotein translocase of trypanosomatids has a bacterial origin. Curr Biol. 2011;21:1738–1743. doi: 10.1016/j.cub.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 76.Requejo R. Hurd TR. Costa NJ. Murphy MP. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 2010;277:1465–1480. doi: 10.1111/j.1742-4658.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santhamma KR. Bhaduri A. Characterization of the respiratory chain of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1995;75:43–53. doi: 10.1016/0166-6851(95)02510-3. [DOI] [PubMed] [Google Scholar]
- 78.Schlecker T. Schmidt A. Dirdjaja N. Voncken F. Clayton C. Krauth-Siegel RL. Substrate specificity, localization, and essential role of the glutathione peroxidase-type tryparedoxin peroxidases in Trypanosoma brucei. J Biol Chem. 2005;280:14385–14394. doi: 10.1074/jbc.M413338200. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt H. Krauth-Siegel RL. Functional and physicochemical characterization of the thioredoxin system in Trypanosoma brucei. J Biol Chem. 2003;278:46329–46336. doi: 10.1074/jbc.M305338200. [DOI] [PubMed] [Google Scholar]
- 80.Silva TM. Peloso EF. Vitor SC. Ribeiro LH. Gadelha FR. O2 consumption rates along the growth curve: New insights into Trypanosoma cruzi mitochondrial respiratory chain. J Bioenerg Biomembr. 2011;43:409–417. doi: 10.1007/s10863-011-9369-0. [DOI] [PubMed] [Google Scholar]
- 81.Smith K. Opperdoes FR. Fairlamb AH. Subcellular distribution of trypanothione reductase in bloodstream and procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol. 1991;48:109–112. doi: 10.1016/0166-6851(91)90170-b. [DOI] [PubMed] [Google Scholar]
- 82.Tetaud E. Giroud C. Prescott AR. Parkin DW. Baltz D. Biteau N. Baltz T. Fairlamb AH. Molecular characterisation of mitochondrial and cytosolic trypanothione-dependent tryparedoxin peroxidases in Trypanosoma brucei. Mol Biochem Parasitol. 2001;116:171–183. doi: 10.1016/s0166-6851(01)00320-6. [DOI] [PubMed] [Google Scholar]
- 83.Tielens AG. van Hellemond JJ. Surprising variety in energy metabolism within Trypanosomatidae. Trends Parasitol. 2009;25:482–490. doi: 10.1016/j.pt.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Turrens JF. Possible role of the NADH-fumarate reductase in superoxide anion and hydrogen peroxide production in Trypanosoma brucei. Mol Biochem Parasitol. 1987;25:55–60. doi: 10.1016/0166-6851(87)90018-1. [DOI] [PubMed] [Google Scholar]
- 85.Vercesi AE. Bernardes CF. Hoffmann ME. Gadelha FR. Docampo R. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem. 1991;266:14431–14434. [PubMed] [Google Scholar]
- 86.Verner Z. Cermáková P. Skodová I. Kriegová E. Horváth A. Lukes J. Complex I (NADH:ubiquinone oxidoreductase) is active in but non-essential for procyclic Trypanosoma brucei. Mol Biochem Parasitol. 2011;175:196–200. doi: 10.1016/j.molbiopara.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Wilkinson SR. Temperton NJ. Mondragon A. Kelly JM. Distinct mitochondrial and cytosolic enzymes mediate trypanothione-dependent peroxide metabolism in Trypanosoma cruzi. J Biol Chem. 2000;275:8220–8225. doi: 10.1074/jbc.275.11.8220. [DOI] [PubMed] [Google Scholar]
- 88.Wilkinson SR. Meyer DJ. Taylor MC. Bromley EV. Miles MA. Kelly JM. The Trypanosoma cruzi enzyme TcGPXI is a glycosomal peroxidase and can be linked to trypanothione reduction by glutathione or tryparedoxin. J Biol Chem. 2002;277:17062–17071. doi: 10.1074/jbc.M111126200. [DOI] [PubMed] [Google Scholar]
- 89.Wilkinson SR. Prathalingam SR. Taylor MC. Ahmed A. Horn D. Kelly JM. Functional characterisation of the iron superoxide dismutase gene repertoire in Trypanosoma brucei. Free Radic Biol Med. 2006;40:198–209. doi: 10.1016/j.freeradbiomed.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L. Yu L. Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998;273:33972–33976. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]