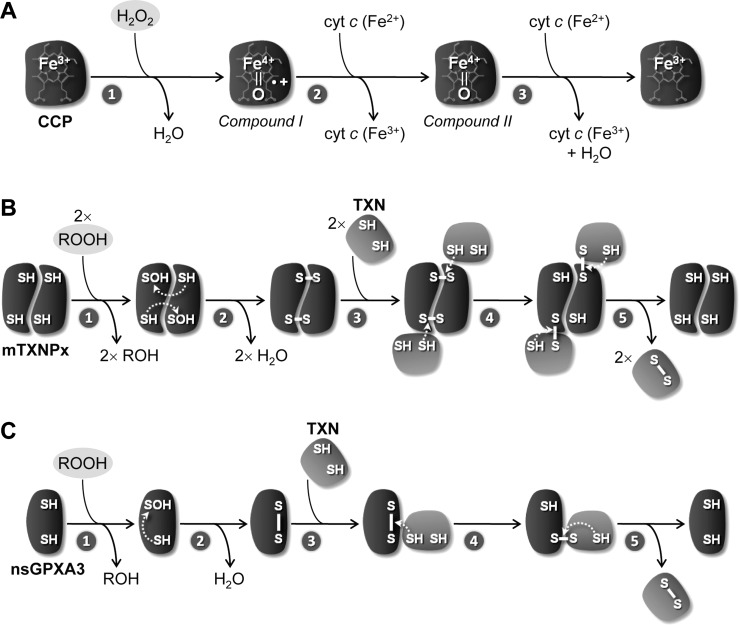
FIG. 6.
Catalytic mechanism of cytochrome c peroxidase and of thiol peroxidases. (A) 1. In the first step of cytochrome c peroxidase (CCP) catalysis, the enzyme reacts with H2O2 in a two-electron redox reaction that leads to the heterolytic cleavage of the hydroperoxide and the release of a molecule of water. In the resulting oxidized enzyme (Compound I), the second H2O2-derived oxygen atom oxidizes the heme iron to a Fe4+=O oxyferryl group and a nearby tryptophan residue to a cationic radical (•+). 2. This radical is subsequently reduced by one molecule of ferrous cyt c [cyt c (Fe2+)] to generate Compound II, which maintains the Fe4+=O center. 3. The enzyme is reduced back to the resting ferric state by a second one-electron transfer from another cyt c (Fe2+) molecule, with release of water. (B) and (C) The thiol peroxidases mTXNPx and nsGPXA3 share the same steps of catalysis, with the difference that the former reacts as a dimer and the latter as a monomer. 1. First, the proximal Cys of the peroxidase reacts with ROOH to yield ROH and sulfenic acid (-SOH). 2. A disulfide bond is then formed inter- (in the case of mTXNPx) or intramolecularly (for nsGPXA3) between the oxidized Cys and a distal thiol group, with concomitant release of water. 3. The disulfide is subsequently attacked by a Cys thiol group from tryparedoxin (TXN). 4. A catalytic intermediate is generated, in which the proximal Cys of the peroxidase is restored to its reduced (-SH) state and the distal Cys is covalently bound to TXN via an S-S bridge. 5. The reaction cycle is completed by a thiol-disulfide exchange reaction, in which the mixed disulfide is resolved by the second Cys residue of TXN, thus releasing the fully reduced peroxidase and the reducing substrate in its oxidized (S-S) form.