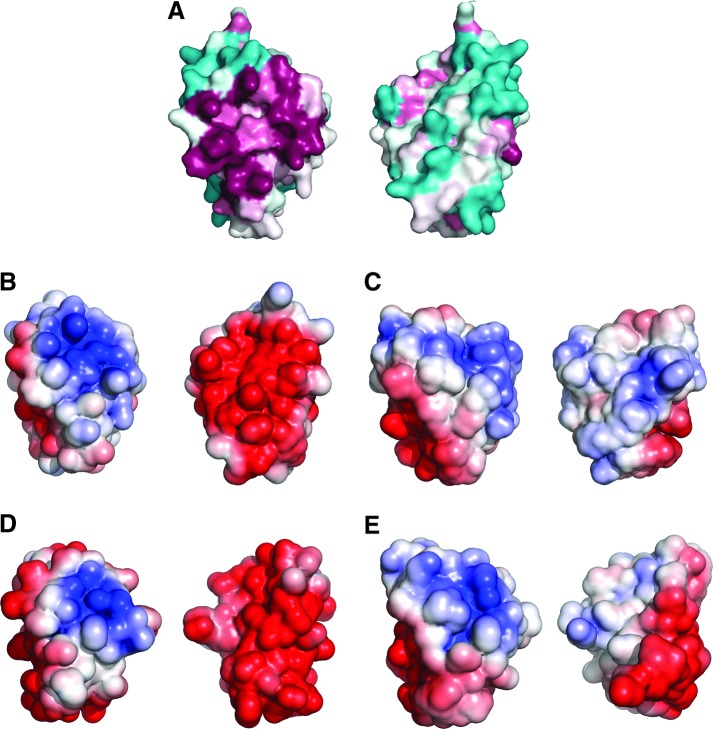
FIG. 6.
Molecular surface of T. brucei 1-C-Grx1 and 3. (A) Surface amino acid conservation in T. brucei 1-C-Grx1 calculated with the Consurf server using the 150 unique 1-C-Grx sequences with the lowest E-value from PSI-BLAST. Conserved and variable residues are depicted in purple and cyan, respectively. Electrostatic potential mapped onto the molecular surface of (B) T. brucei 1-C-Grx1, (C) T. brucei C-terminal domain of Grx3, (D) S. cerevisiae Grx5, and (E) E. coli Grx4; red and blue denote negatively and positively charged residues, respectively. Each image shows two views of the same protein rotated by z-180°. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars