Figure 1.
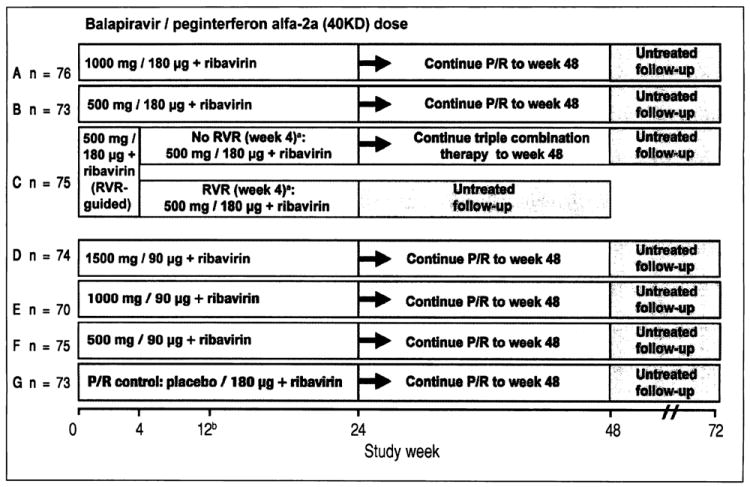
Study design including the planned number of patients in each treatment group. aTherapy was stopped at week 24 in patients with an RVR in group C, provided that their HCV RNA had remained undetectable at all visits through to week 22. bA protocol amendment on May 29, 2008 specified that treatment with balapiravir was to be stopped immediately in all patients in group D, and that treatment with balapiravir was to be stopped at week 12 rather than week 24 in all other treatment groups with a corresponding increase in the length of treatment with peginterferon alfa-2a (40KD) plus ribavirin from 24 to 36 weeks. Patients that had completed more than 12 weeks of treatment with balapiravir were required to stop balapiravir immediately and switch to peginterferon alfa-2a (40KD) plus ribavirin to complete 48 weeks of treatment. Balapiravir was given twice daily (bid). Peginterferon alfa-2a (40KD) was given once weekly. Ribavirin was given at a dosage of 1,000 mg/day to patients weighing < 75 kg and 1,200 mg/day to patients weighing ≥ 75 kg. The daily dosage of ribavirin was divided into two, each half was administered bid with food. RVR: undetectable HCV RNA (< 15 IU/mL) in serum at week 4. P/R: Peginterferon alfa-2a (40KD) 180 μg/week + ribavirin 1,000/1,200 mg/day.
