Abstract
Focality in electro-clinical or neuroimaging data often motivates epileptologists to consider epilepsy surgery in patients with medically-uncontrolled seizures, while not all focal findings are causally associated with the generation of epileptic seizures. With the help of Hill's criteria, we have discussed how to establish causality in the context of the presurgical evaluation of epilepsy. The strengths of EEG include the ability to determine the temporal relationship between cerebral activities and clinical events; thus, scalp video-EEG is necessary in the evaluation of the majority of surgical candidates. The presence of associated ictal discharges can confirm the epileptic nature of a particular spell and whether an observed neuroimaging abnormality is causally associated with the epileptic seizure. Conversely, one should be aware that scalp EEG has a limited spatial resolution and sometimes exhibits propagated epileptiform discharges more predominantly than in situ discharges generated at the seizure-onset zone. Intraoperative or extraoperative electrocorticography (ECoG) is utilized when noninvasive presurgical evaluation, including anatomical and functional neuroimaging, fails to determine the margin between the presumed epileptogenic zone and eloquent cortex. Retrospective as well as prospective studies have reported that complete resection of the seizure-onset zone on ECoG was associated with a better seizure outcome, but not all patients became seizure-free following such resective surgery. Some retrospective studies suggested that resection of sites showing high-frequency oscillations (HFOs) at >80 Hz on interictal or ictal ECoG was associated with a better seizure outcome. Others reported that functionally-important areas may generate HFOs of a physiological nature during rest as well as sensorimotor and cognitive tasks. Resection of sites showing task-related augmentation of HFOs has been reported to indeed result in functional loss following surgery. Thus, some but not all sites showing interictal HFOs are causally associated with seizure generation. Furthermore, evidence suggests that some task-related HFOs can be transiently suppressed by the prior occurrence of interictal spikes. The significance of interictal HFOs should be assessed by taking into account the eloquent cortex, seizure-onset zone, and cortical lesions. Video-EEG and ECoG generally provide useful but still limited information to establish causality in presurgical evaluation. A comprehensive assessment of data derived from multiple modalities is ultimately required for successful management.
Keywords: infantile spasms, pediatric epilepsy surgery, concept of the epileptogenic zone, eloquent cortex, functional brain mapping, language, in-vivo animation of event-related gamma activity, Hill's criteria for causality, high-frequency oscillations (HFOs)
Introduction
Regardless of patient age, the ultimate goal of epilepsy surgery is to eliminate the recurrence of epileptic seizures while not creating a new sensory, motor, or cognitive deficit. The first step in presurgical evaluation is to determine whether the habitual spells are indeed epileptic seizures of focal origin. The second step is to localize the presumed epileptogenic zone using a multimodality approach. The epileptogenic zone has been conceptually defined as the region of which resection is necessary and sufficient to achieve seizure-freedom (Table 1) [1, 2]. Thus, the true physical extent of the epileptogenic zone cannot be directly measured, but it can be estimated by epileptologists using their own, often individually developed, criteria (Table 1). The third step is to determine the spatial relationship between the presumed epileptogenic zone and eloquent cortex, as can be defined by various functional brain mapping modalities (Table 1). The final step is to determine whether and how the presumed epileptogenic zone can be completely removed without sacrificing eloquent cortex (Figure 1).
Table 1.
Revisiting the concepts of the epileptogenic zone (modified from Lüders and Awad [1]).
| Cortical zones | Descriptions | Modalities utilized for localization |
|---|---|---|
| Epileptogenic zone | Region of which resection is necessary and sufficient to achieve seizure-freedom. | Video; EEG; ECoG; MEG; MRI; PET; Ictal SPECT; Postoperative photograph of brain; Postoperative MRI; Postoperative seizure outcome. |
| Irritative zone | Region generating interictal epileptiform discharges. | EEG; ECoG; MEG. |
| Seizure onset zone | Region initially generating ictal discharges. | EEG; ECoG. |
| Symptomatogenic zone | Region responsible for generating ictal symptoms | Video; EEG; ECoG; Electrical stimulation; MRI. |
| Epileptogenic lesion | Structural lesion, which is causally associated with epilepsy. | MRI; Video; EEG; ECoG; MEG; Postoperative seizure outcome. |
| Functional deficit zone | Region functionally abnormal during interictal state. | PET; SPECT. |
| Eloquent cortex | Region essential for a given sensorimotor or cognitive function. | Wada test; Electrical stimulation; ECoG; MEG; functional MRI; Postoperative behavioral outcome. |
“Epileptogenic zone” is sufficiently included in the resection cavity seen on a postoperative photograph or MRI in a patient who has achieved long-term seizure-freedom following resective surgery. Still, the extent of the epileptogenic zone cannot be accurately measured since the region necessary to obtain seizure-freedom remains uncertain. In practice, investigators at each epilepsy center, often using their own combination of modalities and criteria, determine the “presumed epileptogenic zone” to be removed. In our previous study using electrocorticography (ECoG), for example, we defined the presumed epileptogenic zone as the summation of the seizure onset zone, the neighboring cortical lesion(s), and the neighboring region(s) showing frequent interictal epileptiform discharges [3]. It is unknown whether the presumed epileptogenic zone indeed needs to be completely removed to achieve seizure-freedom. This is further complicated by the fact that complete resection of the presumed epileptogenic zone does not always result in complete seizure freedom.
“Interictal epileptiform discharges” consist of spikes, polyspikes, and sharp waves on EEG, ECoG, or magnetoencephalography (MEG). Sharp transients or sharply-contoured waves explainable as physiological activities are not considered as interictal epileptiform discharges [4, 5].
“Ictal discharges” are defined here as sustained, rhythmic electrographic discharges accompanied by subsequent clinical habitual seizure symptoms; not simply explained by state changes and clearly distinguished from background and interictal electrographic activity [3, 6, 7]. Exceptions to this definition include myoclonic seizures and epileptic spasms, of which ictal discharges may be much shorter than 1 second in total duration [8, 9].
“Symptomatogenic zone” can be defined as the region of which electrographic involvement or electrical stimulation elicits a given ictal symptom. For example, the symptomatogenic zone for seizures characterized by left upper extremity jerking is estimated to be localized in the right pre- and post-central gyri [9]. Eloquent cortex, also known as functionally necessary cortex, is often a part of the symptomatogenic zone [2].
“Epileptogenic lesion” was originally defined as the MRI-visible lesion causally related to the epileptic pathophysiology [1, 2]. Proof of such a causal relationship would require further consideration of convergence of a given lesion with epileptiform discharges, semiology, or postoperative seizure outcome. In this review article, we have used the term: “cortical lesion” instead of “epileptogenic lesion”, in cases where the causal relationship between a given lesion and the epileptogenic process is not proven. Table 2 presents criteria to consider before concluding that a given cortical lesion is likely to be causal.
“Functional deficit zone” is commonly delineated as hypometabolic regions on 2-deoxy-2-[18F]fluoroglucose-positron emission tomography (FDG-PET) imaging. A pathologic specimen of a functional deficit zone may contain focal cortical dysplasia in some epileptic patients without lesions identifiable on MRI [10–11]. The interictal single photon emission computed tomography (SPECT) scan is less frequently utilized to delineate the functional deficit zone, partly due to poor spatial resolution.
Figure 1. Possible scenarios of epilepsy surgery.
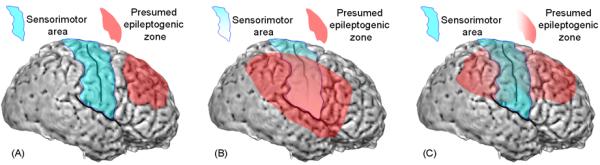
(A) The illustration shows a case in which the presumed epileptogenic zone (red) is localized to a region distant from eloquent cortex (blue). The primary intent of a surgeon would be to maximize removal of the presumed epileptogenic zone while preserving eloquent cortex. The symptomatogenic zone is dependent on the propagation pattern of ictal discharges. The sensorimotor cortex here may be considered as the symptomatogenic zone if the propagation of ictal discharges to it resulted in seizure-related symptoms characterized by jerking of the contralateral body.
(B) The illustration shows a case in which the presumed epileptogenic zone involves a large portion of eloquent cortex also serving as the symptomatogenic zone. The surgeon's intent would be to remove the symptomatogenic zone together with the presumed epileptogenic zone; for example, hemispherectomy including the sensorimotor area is a likely approach. The resulting functional losses due to hemispherectomy would include visual field loss, if the function was intact preoperatively, and a variable degree of enhancement in sensorimotor deficits.
(C) The illustration shows a case in which the presumed epileptogenic zone involves, to a lesser degree, eloquent cortex. This scenario is the case, for example, when the seizure onset zone is localized outside of eloquent cortex but very frequent interictal spike-wave discharges involve the eloquent cortex. One approach would be partial removal of the presumed epileptogenic zone along with maximal preservation of the eloquent cortex. An alternative approach would be maximal removal of the presumed epileptogenic zone together with eloquent cortex (serving as the symptomatogenic zone). With the later approach, a greater chance of seizure-freedom is plausible since resection includes the symptomatogenic zone responsible for generating the ictal symptom; the drawback of this more aggressive approach is a greater chance of development of a new or further enhancement of an existing functional deficit.
Causality criteria
The expectation that a patient is likely to benefit from epilepsy surgery is often motivated by focality in electro-clinical or neuroimaging data. However, not all focal findings are causally associated with the generation of epileptic seizures. In this article, we discuss how to judge causality in the context of the presurgical evaluation of epilepsy by incorporating Hill's nine criteria for causation (Table 2) [12]. In Figures 2 and 3, we have demonstrated two representative examples highlighting how one may establish a causal relationship between focal imaging abnormalities and seizure generation in individual patients (Tables 3 and 4). The roles of video-EEG and intracranial electrocorticography (ECoG) are described in this context below.
Table 2.
Guidelines for judging whether a relationship is causal (modified from Hill [12]).
| Criterion to judge if Event [A] causes Outcome [B]. | Definition | Example: How can we establish a causal relationship between epilepsy surgery and cognitive improvement in children? |
|---|---|---|
| Temporal relationship | The causal event must precede the outcome; lack of temporal ambiguity. | Epilepsy surgery must precede cognitive improvement to prove the causal relationship. |
| Strength of association | A strong association suggests a higher likelihood of causality. A 95% confidence interval, if relevant, may be helpful in assessing this criterion. | A much larger degree of cognitive improvement in a surgical group compared to a non-surgical group would suggest that the association is causal. |
| Biologic gradient (dose-response effect) | Correlation between the amount of events and the outcome measures in a predicted direction supports the causal relationship conclusion. | Correlation between the post-operative reduction in seizure frequency and the degree of cognitive improvement or correlation between more delayed surgery and more severe cognitive dysfunction would support the causal nature of surgery for good cognitive outcomes. |
| Replication of findings | A causal relationship is more strongly supported if the association-of-interest is consistently observed in other cohorts by other investigators at other institutions. | A single study of children with temporal lobe epilepsy reported that a postoperative intellectual outcome was better in the surgical than in the non-surgical group [13]. Confirmatory observations by other groups would more strongly support a causal relationship. |
| Coherence (consistency with other knowledge) | A causal conclusion should not fundamentally contradict generally-accepted knowledge. | The causal relationship between seizure control by epilepsy surgery and subsequent cognitive improvement sounds consistent with the generally-accepted notion that seizures may negatively impact physiological brain function as well as social conditions. |
| Biological plausibility | One should consider if the proposed causal relationship is plausible, based on current knowledge of the underlying biology. | Since many anti-epileptic medications are known to reduce the excitability of individual neurons through various mechanisms, it is plausible to suspect that such medications may cause unwanted global cognitive side-effects. Conversely, one must consider the functional deficits that may result from the sudden removal of vast cortical networks due to epilepsy surgery. |
| Analogy | A causal relationship is more likely when the event-of-interest is a known cause of another related condition or a similar event has a causal effect on the condition-of-interest. | If a non-surgical therapy for seizure control already shows strong evidence to be causally related to cognitive improvement, the causal relationship between epilepsy surgery and cognitive improvement becomes more likely. |
| Specificity | If the event is specific to the outcome, causation is more likely. | If epilepsy surgery, but none of the other therapies, is associated with cognitive improvement, the causal relationship between epilepsy surgery and cognitive improvement becomes more likely. |
| Experimental evidence | If evidence is based on randomized, controlled experiments or if a preventative intervention alters the outcome, causation is more likely. | The observation of better cognitive improvement in the surgery group compared to the non-surgical group in a randomized, controlled prospective study strongly suggests that the relationship between epilepsy surgery and cognitive improvement is causal. |
It is recommended to consider and weigh the criteria against each other when attempting to infer a causal relationship between an event and an outcome. A causal relationship does not have to satisfy all of the criteria. A biological gradient or analogy may not logically exist in some clinical scenarios. Coherence and biological plausibility are certainly dependent on the current knowledge of a disease or treatment. While negative results or surgical failure could be under-reported, it is difficult to estimate the severity of such publication bias [14]. It is also recommended to consider whether an event-of-interest is the direct or indirect cause of the outcome. For example, it is possible to hypothesize that epilepsy surgery could be the direct cause of reduced antiepileptic medications and that reduced antiepileptic medications could be the direct cause of cognitive improvement; thus, epilepsy surgery might then be an indirect cause of cognitive improvement. Causal relationships between pediatric epilepsy surgery and cognitive improvement remain to be established by a randomized, controlled prospective study. Previous case-series and case-control studies reported that the strengths of associations between pediatric epilepsy surgery and cognitive improvement have ranged from `undetectable' to `significant but modest' [13, 15–17], but this does not necessarily disprove a causal relationship. Each individual criterion should be considered as a piece of evidence, rather than as proof, for or against causation; the accumulated knowledge of the aggregate set of criteria is what should influence the conclusion.
Figure 2. Removal of the presumed epileptogenic zone while preserving eloquent cortex.
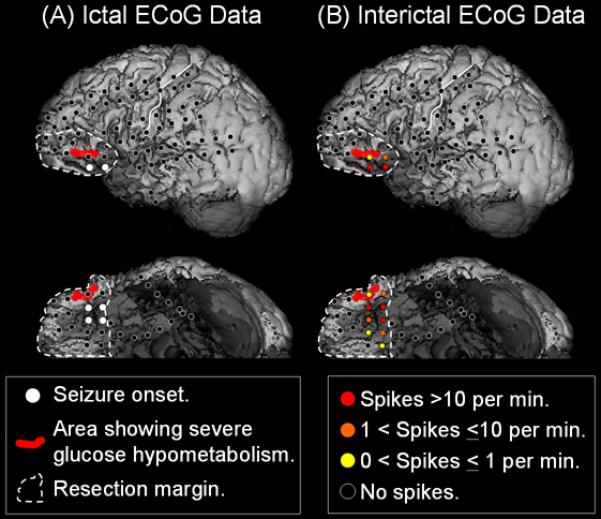
A 9-year-old, right-handed girl with focal epilepsy and normal MRI underwent extraoperative ECoG. Her habitual seizures were characterized by sudden-onset and brief hypermotor automatism followed by minimal postictal confusion. Both seizure onset and irritative zones were localized to the ventral surface of the left frontal lobe on ECoG [3]. While MRI failed to show a cortical lesion, FDG-PET showed severe glucose hypometabolism near the seizure onset zone in the ventral surface of the left frontal lobe [18]. Glucose metabolism in the red-shaded area was >15% lower than that of the contralateral homotopic region [3]. Electrical stimulation of the seizure onset zone elicited her habitual seizures. The intent of cortical resection was to completely remove the presumed epileptogenic zone while preserving the sensorimotor and language cortices, as suggested by electrical stimulation and anatomical landmarks. After surgery, pathological examination revealed focal cortical dysplasia; she has been seizure-free without a new cognitive deficit for 6 years. The epileptogenic zone is included within the resection cavity, the margin of which is denoted by broken lines, but it remains unknown if this extent of resection was necessary to achieve long-term seizure-freedom. As demonstrated in Table 3 below, it was reasonably straightforward to establish the causal relationship between imaging abnormalities of the ventral surface of the left frontal lobe and generation of epileptic seizures in this patient.
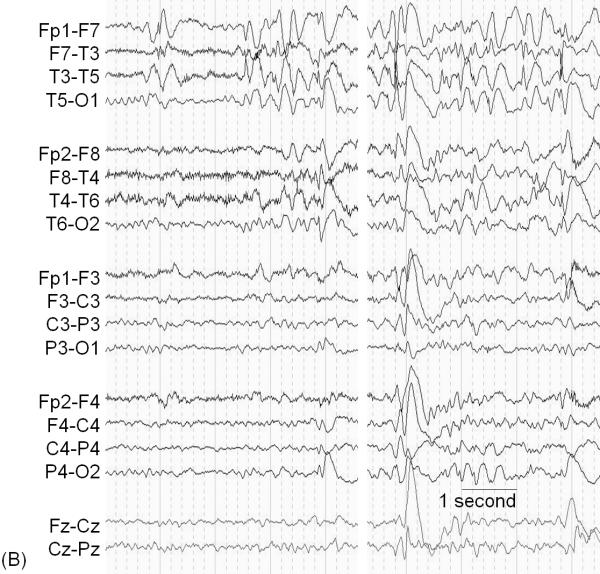
Figure 3. Removal of the presumed epileptogenic zone along with eloquent cortex.
A 7-year-old, right-handed girl with a history of intractable epilepsy associated with Sturge-Weber syndrome underwent epilepsy surgery. A port wine stain was noted on her right face shortly after birth; seizures developed by 11 months of age. Before surgery, she exhibited mild hemiparesis as well as a left-sided visual field deficit. Her habitual seizures were characterized by behavioral arrest, transient worsening of hemiparesis on the left side, as well as occasional generalized tonic-clonic convulsions. (A) MRI showed severe atrophy of the right hemisphere involving the right Rolandic cortex (arrow). (B) Ictal EEG (arrowhead) showed sustained rhythmic slow wave discharges mixed with low-amplitude fast waves maximally involving the left posterior head region; seizure-onset is marked by the black arrowhead. High-pass filter cutoff: 1.6 Hz. Low-pass filter cutoff: 30 Hz. The intraoperative photograph showed diffuse angiomatosis involving the right hemisphere. Prior to surgery, we concluded the probable causal relationship between the broad right-hemispheric lesion and epilepsy (Table 4). The intent was to completely remove the presumed epileptogenic zone including the right Rolandic cortex by anatomical hemispherectomy [19]. Following surgery, our presumed causal relationship was confirmed by her ongoing seizure freedom, but she has suffered a worsening of her left hemiparesis, which required physical therapy.
Table 3.
Judgment of causality in a 9-year-old girl with focal epilepsy and normal MRI.
| Criterion to judge causality | How can we conclude a causal relationship between glucose hypometabolism involving the ventral frontal lobe and seizure generation in this case (Figure 2)? |
|---|---|
| Temporal relationship | Focal ictal discharges on ECoG, originating from the ventral frontal region, preceded the onset of clinical seizures. |
| Strength of association | Ictal discharges arose from the same region proximal to the glucose hypometabolic region in all seizure events. |
| Biologic gradient (dose-response effect) | The seizure onset zone proximal to the glucose hypometabolic region showed the largest rate of interictal epileptiform discharges on ECoG, whereas more distant regions showed less frequent interictal epileptiform discharges. |
| Replication of findings | Other groups reported that complete resection of a focal hypometabolic area in patients with cortical dysplasia resulted in a good seizure outcome [20]. |
| Coherence (consistency with other knowledge) | Other groups reported that hypometabolic areas proximal to the seizure onset zone often contain focal cortical dysplasia [21]. |
| Biological plausibility | Focal glucose hypometabolism can be explained by neuronal loss or an abnormal dendritic pattern of dysplastic neurons [22, 23]. |
| Analogy | Patients with an MRI lesion in this location can have brief seizures with hypermotor automatisms [24, 25]. |
| Specificity | Such glucose hypometabolism is rare in healthy individuals [26]. Seizures characterized by brief hypermotor semiology are reported to be seen most frequently in patients with frontal lobe epilepsy and less frequently in those with other forms of focal epilepsy [24, 25]. |
| Experimental evidence | Electrical stimulation of the seizure onset zone proximal to the glucose hypometabolic region induced her habitual seizures. Cortical resection indeed resulted in seizure-freedom. |
Table 4.
Judgment of causality in a 7-year-old girl with Sturge-Weber syndrome.
| Criterion to judge causality | How can we conclude a causal relationship between the right-hemispheric lesion and seizure generation in this patient, whose seizure onset on scalp EEG appeared left-sided (Figure 3)? |
|---|---|
| Temporal relationship | The cerebral lesion was likely present prior to the onset of the first seizure, due to the nature of underlying disease. Ictal discharges on scalp EEG preceded the onset of clinical seizure symptoms. |
| Strength of association | Convergence between the right-hemispheric lesion and semiology (ictal hemiparesis on the left side) was confirmed. |
| Biologic gradient (dose–response effect) | Not relevant in this scenario. |
| Replication of findings | Other groups reported that resection of large and unilateral early-onset lesions resulted in good seizure outcomes, despite EEG abnormalities involving the contralateral hemisphere [27, 28]. |
| Coherence (consistency with other knowledge) | Transient worsening of left hemiparesis during seizure events was consistent with the notion that ictal discharges originated from the right hemisphere. |
| Biological plausibility | False lateralization of seizure onset on scalp EEG can be partly explained by the effect of cortical atrophy on the right hemisphere; cortical atrophy increased the distance between the generator of epileptiform discharges and recording electrodes and, in turn, decreased the apparent amplitude of ictal discharges in the atrophic hemisphere. |
| Analogy | Complete resection of massive brain malformations of unilateral involvement (such as porencephaly, ulegyria, and dysplasia) as well as hypothalamic hamartoma was reported to result in good seizure control, regardless of scalp EEG findings [28, 29]. |
| Specificity | Ictal hemiparesis has been specifically reported in patients with a cortical lesion or seizure onset zone involving the Rolandic region [30, 31]. Thus, it was plausible to expect that the presumed epileptogenic zone involved the right primary sensorimotor cortex in this case. |
| Experimental evidence | We did not have experimental evidence prior to surgery. The observation of seizure control following right-sided hemispherectomy suggests a causal relationship between the right-hemispheric lesion and generation of epileptic seizures, in this case. |
Role of scalp video-EEG monitoring
The strengths of EEG recording include low operating cost and the ability to determine the temporal relationship between cerebral activities and clinical events. Scalp video-EEG is generally necessary but not sufficient in the evaluation of the majority of surgical candidates. Ictal EEG discharges temporally associated with clinical spells, if present, can confirm that such spells are indeed epileptic in nature. Previous studies reported that 15–43% of children originally suspected to be experiencing epileptic seizures were finally considered to exhibit paroxysmal non-epileptic events, primarily based upon assessment with scalp video-EEG data [32–35]. Convergence in spatial localization between a cortical lesion and ictal EEG discharges is one piece of evidence justifying the assumption that the lesion is causally related to the epileptic pathophysiology (Table 1). When such imaging-EEG convergence is not present (see an example in Figure 4 and Table 5), further consideration of other criteria for causality would be recommended before predicting a good seizure outcome following resection of the cortical lesion.
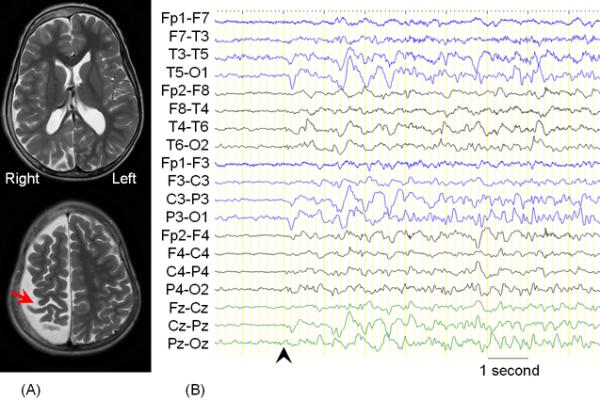
Figure 4. Removal of a cortical lesion not causally related to the generation of epileptic seizures.
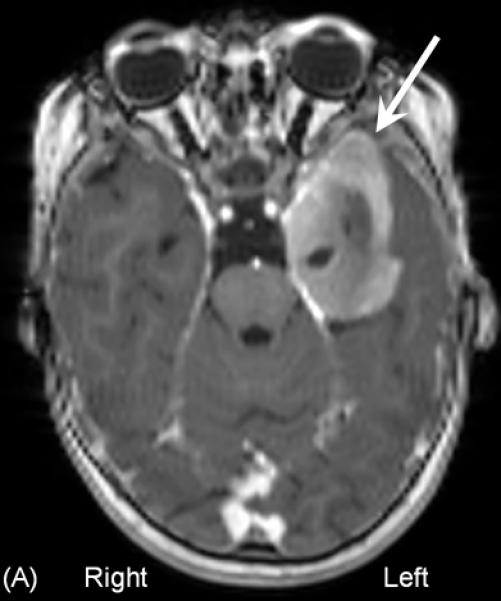
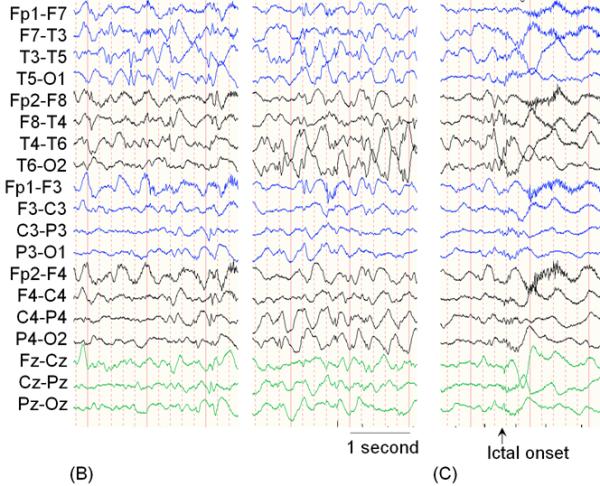
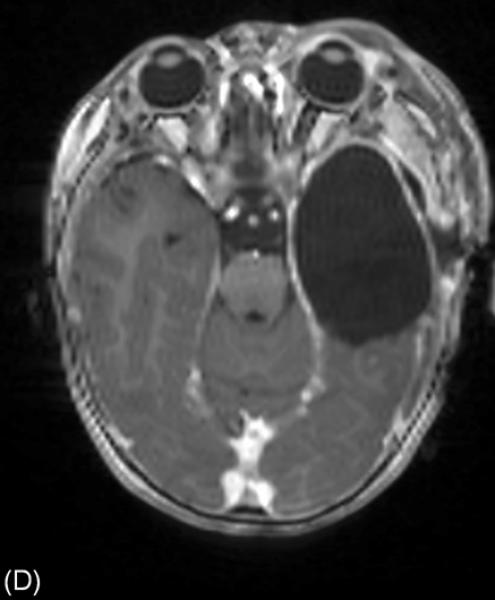
A 16-month-old girl with a history of intractable epileptic spasms associated with a brain tumor and Down syndrome underwent epilepsy surgery. She developed epileptic spasms at 6 months of age. (A) MRI showed a left temporal lobe tumor with gadolinium enhancement (arrow). (B) Interictal scalp video-EEG showed independent spike-wave discharges arising from the left and right temporal regions as well as posterior head regions, independently. Generalized spike-wave discharges were also noted, and some of the sleep EEG segments showed hypsarrhythmia (not shown here). A high-pass filter cutoff of 1.6 Hz and a low-pass filter cutoff of 70 Hz were applied. (C) Ictal EEG associated with each spasm event showed diffuse, low-amplitude, fast wave bursts without a consistent leading wave in the left hemisphere. The aforementioned data failed to suggest a causal relationship between the cortical lesion in the left temporal lobe and generation of epileptic spasms (Table 5). Our surgical intention was complete removal of the brain tumor and the surrounding tissues. Intraoperative electrocorticography (ECoG) showed multifocal interictal epileptiform discharges in the left temporal and extratemporal regions. Postoperative ECoG following a left temporal lobectomy that included the brain tumor continued to show interictal epileptiform discharges in the extratemporal region. (D) Postoperative MRI confirmed successful resection of the brain tumor. However, postoperative scalp EEG showed multifocal and independent interictal spike-wave discharges bilaterally and the patient continued to have epileptic spasms. Pathology revealed a high-grade glioma.
Table 5.
Judgment of causality in a 16-month-old girl with epileptic spasms associated with a brain tumor.
| Criterion to judge causality | How can we conclude a causal relationship between the left temporal tumor and seizure generation in this case (Figure 4)? |
|---|---|
| Temporal relationship | It is unknown if the tumor was present prior to the onset of the first seizure, whereas the patient was born with Down syndrome. Ictal discharges on scalp EEG preceded the onset of clinical seizure symptoms. |
| Strength of association | We failed to observe spatial convergence between the left temporal lobe tumor and epileptiform discharges on scalp EEG. |
| Biologic gradient (dose-response effect) | Visual assessment of scalp EEG recording failed to suggest a correlation between the distance from the left temporal lobe tumor and the rate or amplitude of interictal or ictal epileptiform discharges. |
| Replication of findings | Other groups reported that resection of large, unilateral developmental lesions resulted in good seizure outcomes, despite EEG abnormalities involving the contralateral hemisphere [27, 28]; however, we did not know preoperatively if the cortical lesion in this patient was a developmental or acquired tumor. |
| Coherence (consistency with other knowledge) | We failed to observe semiology consistent with focal seizures arising from the left hemisphere (e.g.: jerking predominantly involving the right side of the body) or semiology consistent with temporal lobe epilepsy (e.g.: staring or automatism). |
| Biological plausibility | It was difficult to plausibly explain the mechanism of multifocal EEG abnormalities only using the left temporal tumor and the surrounding tissues. Further complicating the matter, there was neither cortical atrophy nor any midline structural lesion which could have induced the apparent contralateral discharges. |
| Analogy | Complete resection of hypothalamic hamartoma was reported to result in good seizure control, regardless of scalp EEG findings [29]. It is uncertain if this is applicable to a temporal lobe tumor which may or may not be a developmental lesion. |
| Specificity | This patient had Down syndrome and left temporal lobe tumor. Thus, it is possible that epileptic spasms in this patient may be causally associated with Down syndrome rather than the brain tumor. |
| Experimental evidence | Continued spasms following left temporal lobectomy including the tumor failed to suggest a causal relationship between the tumor and the generation of epileptic spasms in this case. |
Simultaneous EEG recording during acquisition of positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging is likely to secure a more accurate interpretation of such functional imaging studies. In general, patients with unifocal epilepsy are expected to show focal or regional hypometabolism or hypoperfusion within or near the seizure onset zone, during the interictal state [36]; resection of which may lead to a favorable outcome [37, 38]. Such functional imaging abnormalities generally contribute to noninvasive localization or lateralization of the presumed epileptogenic zone. Yet, PET studies using simultaneous scalp EEG recording reported that very frequent interictal epileptiform discharges, if present, were associated with glucose hypermetabolism in the presumed epileptogenic hemisphere. Cortical resection involving such glucose hypermetabolism was reported to result in a good seizure outcome [10, 39]. A plausible explanation for hypermetabolism on interictal PET is that very frequent interictal spike discharges may transiently increase glucose metabolic demands regionally (e.g.: for maintenance of membrane potentials recurrently altered by spikes) [40, 41]. Further studies remain to determine whether very frequent focal interictal epileptiform discharges are the cause or result of interictal focal glucose hypermetabolism on PET; for now, we know only that they are associated.
Studies of adults and children with temporal and extratemporal lobe epilepsy reported that spatial convergence between the site of resection, assessed by intraoperative photographs or post-operative MRI, and interictal epileptiform discharges on scalp EEG recording predicted a better seizure outcome [42–46]. Conversely, one should be aware that scalp EEG sometimes exhibits propagated epileptiform discharges more predominantly than in situ discharges generated at the seizure-onset zone (Figure 3). Such an unwanted phenomenon is also seen on magnetoencephalography (MEG) [47]. For example, patients with large and unilateral early-onset cortical lesions occasionally show generalized or even falsely lateralized epileptiform discharges on scalp EEG even though complete resection of these lesions often resulted in seizure control (Table 4) [27, 28]. In these patients, therefore, the role of scalp video-EEG recording would be limited to the exclusion of the possibility of non-epileptic events and comparison of epileptiform discharge characteristics between pre- and post-operative periods; although Table 5 and Figure 4 depict a seemingly similar case in which the EEG findings should have been given greater weight in order to accurately predict the postoperative seizure outcome.
The role of scalp video-EEG recording is also limited in patients with hypothalamic hamartoma [29], who may not show ictal epileptiform discharges during gelastic seizures. This absence of ictal discharges on scalp EEG may be attributed to the large distance between recording electrodes and the generator of epileptiform discharges. Based on the observation of a strong and specific association between hypothalamic hamartoma and the generation of gelastic seizures, as well as good seizure outcome following lesionectomy [29, 48], the epileptogenic zone is considered to involve the hypothalamic lesion itself, regardless of a lack of scalp EEG findings.
Role of ECoG monitoring
The purpose of ECoG recording is to refine the presumed physical boundaries of the epileptogenic zone as well as eloquent cortex, with a spatial resolution of 1 cm or below, when such spatial relationships remain unclear after evaluation using noninvasive diagnostic modalities. In our pediatric epilepsy center, we most frequently sample ECoG using subdural grid and strip electrodes (Figure 2) following craniotomy; some other groups prefer depth electrodes implanted stereotactically [49, 50]. The advantage of subdural grid and strip electrodes includes signal sampling from more widespread cortical areas, while that of depth electrodes includes improved signal sampling from deep structures such as hippocampus and gray matter within a deep sulcus [51].
ECoG signals can be sampled intra- and extra-operatively. The advantages of extraoperative ECoG monitoring, as compared to intraoperative, include (i) a greater chance of capturing ictal events, (ii) exclusion of the effect of anesthetic agents on ECoG signals, and (iii) a better opportunity to localize eloquent cortex; these benefits being due to the extended, awake recording that is enabled. Our previous study reported that general anesthesia reduced the rate of interictal epileptiform discharges in a majority of patients with focal epilepsy who exhibited frequent interictal spiking during extraoperative recording [52]. Also, not many children with epilepsy can tolerate awake craniotomy for intraoperative functional language mapping [53]. The disadvantages of extraoperative ECoG monitoring include: (i) a requirement for a second craniotomy, (ii) greater financial cost, and (iii) increased risk of complications, including infection [54]. Accumulated knowledge in epileptology and improvement in anatomical and functional neuroimaging techniques have resulted in a reduced proportion of patients who undergo extraoperative ECoG recording prior to resective surgery. Nonetheless, it is still commonly utilized in a number of epilepsy centers, and investigators continue to search for ECoG biomarkers that may further enhance outcomes following surgery.
Significance of ictal discharges consisting of HFOs on ECoG recording
It is plausible to presume that the region generating the initial ictal discharge on ECoG (Table 1) is part of an epileptogenic zone, at least when all cortical regions are sampled by intracranial electrodes. Although such spatially-complete signal sampling is not feasible (see Figure 2 as an example), a number of prospective and retrospective studies using ECoG reported that complete resection of the seizure onset zone was associated with a better seizure outcome [3, 44, 45, 55]. As an example, resection of cortical tubers involved in the seizure onset zone resulted in good seizure control in a substantial proportion of patients with tuberous sclerosis complex, even though the remaining tubers were left undisturbed [56–58].
It is also plausible to presume that the cortical region associated with ictal activity dominated by higher frequencies, especially ictal discharges consisting of high-frequency oscillations (HFOs), may be a superior biomarker to localize the epileptogenic zone. This was supported by the observation that a localized seizure onset pattern on ECoG frequently consisted of sustained rhythmic oscillations of beta or faster band, while slow rhythmic oscillations of theta or delta band were noted in surrounding or remote regions [59]. A lack of sustained oscillations of beta or faster band at the ictal onset has been associated with poorer seizure outcome following resective surgery [7, 60].
Technological improvements in acquisition and data storage systems have recently allowed us to continuously record ECoG with a sampling rate of >1000 Hz and to assess the spatiotemporal dynamics of HFOs of higher spectral frequency prior to and during ictal events. A number of ECoG studies have reported the presence of ictal HFOs at >60 Hz during focal seizures [61–64]; there was an impressive convergence between the locations showing ictal and interictal HFOs >80 Hz [65], and more complete resection of the sites showing ictal HFOs >60 Hz was associated with a better seizure outcome [66]. We have also studied ictal HFOs seen in 636 events of epileptic spasms [9] and found ictal HFOs at 80–200 Hz to be causally associated with the generation of epileptic spasms (Figure 5 and Table 6).
Figure 5. High-frequency oscillations (HFOs) during epileptic spasms (modified from [95]).
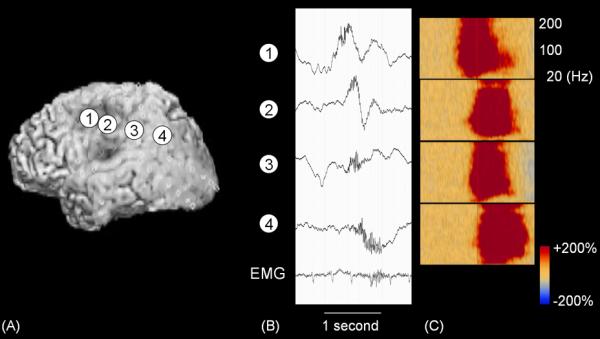
(A) A 30-month-old boy with a history of intractable epileptic spasms underwent implantation of subdural electrodes over the left hemisphere. (B) Ictal HFOs were initially generated by the left frontal region and propagated to other sites. High-pass filer cutoff: 0.08 Hz. Low-pass filter cutoff: 300 Hz. (C) Time-frequency analysis showed that augmentation of HFOs at 80–200 Hz preceded those at beta range.
Table 6.
Judgment of causality between ictal high-frequency oscillations (HFOs) and generation of epileptic spasms.
| Criterion to judge causality | How can we conclude a causal relationship between ictal HFOs and generation of epileptic spasms? |
|---|---|
| Temporal relationship | Augmentation of ictal HFOs preceded the clinical onset of epileptic spasms [9]. |
| Strength of association | All spasm events were associated with augmentation of HFOs on ECoG [9, 67]. |
| Biologic gradient (dose-response effect) | The presence of arm jerking recorded on electromyography was associated with greater augmentation of ictal HFOs in the sensorimotor hand area on the contralateral side [9]. |
| Replication of findings | Using scalp EEG and ECoG, other groups reported ictal augmentation of HFOs at >50 Hz preceding the clinical onset of epileptic spasms [68–70]. |
| Coherence (consistency with other knowledge) | Ictal HFOs often originated from the irritative zone, cortical lesion on MRI, or glucose hypometabolic region on PET [67–69]. |
| Biological plausibility | Ictal augmentation of HFOs can be explained by increased spiking in pyramidal cells, interneurons or both [71, 72]. One must also consider the possibility of spatial sampling error in each surgical case; initial HFOs on a channel could be propagated activities from an unsampled site. |
| Analogy | Removal of sites showing ictal HFOs at 60–100 Hz at the onset of focal seizures was reported to result in a good seizure outcome [66]. |
| Specificity | Augmentation of HFOs at 80–200 Hz was greater in amplitude and earlier in onset latency, compared to those at beta range [9]. Such augmentation of HFOs occurred just prior to and during spasms. |
| Experimental evidence | Removal of the site showing initial augmentation of ictal HFOs was associated with a better seizure outcome [9], though the sample size of this retrospective study was small. |
Significance of interictal spikes and HFOs on ECoG recording
Since ictal events are not always available for review, we previously attempted to predict the location of the seizure onset zone, and also surgical outcome, using only the rate of interictal epileptiform discharges, consisting of spikes, polyspikes, and sharp waves on ECoG. Retrospective studies of children and adults with focal epilepsy using extraoperative and intraoperative ECoG reported that the site showing the most frequent rate of interictal epileptiform discharges were frequently located within or close to the seizure onset zone [52, 73–75]. Our prospective study of 61 patients using extraoperative ECoG reported that a lower rate of interictal epileptiform discharges in the preserved cortex was associated with a better seizure outcome, but multivariate analysis suggested that complete resection of the actual seizure onset zone was the only independent predictor of seizure freedom [3]. Taken together, we have failed to demonstrate that the rate of interictal spikes on ECoG, at least when acquired with a sampling frequency of only 200 Hz, can replace ictal ECoG recording.
Recently, as a potential alternative biomarker to interictal spikes, spontaneously generated HFOs at > 80 Hz on interictal ECoG recording have attracted growing interest (Figure 6), since several retrospective studies of adults and children with focal epilepsy reported that a greater rate of interictal HFOs at >80 Hz was noted within the seizure onset zone [76–78] as well as atrophic hippocampus [79], compared to other apparently uninvolved control regions. A more complete resection of sites showing interictal HFOs was associated with a better seizure outcome [50, 77, 80]. The outcome prediction performance of the retrospectively-determined rate of HFOs seemed to be better than that of the seizure onset zone; with the latter being prospectively determined upon ictal-recording primarily acquired with a filter setting of 0.3–70 Hz [50, 65]. The aforementioned observations provide evidence supporting a causal relationship between interictal HFOs at >80 Hz and the generation of epileptic seizures (Table 7). Yet, it should be noted that HFOs at >80 Hz are episodically or continuously generated by eloquent cortex such as visual and motor areas (Figure 6) as well as non-epileptogenic medial temporal lobe regions, even during sleep [78, 85–87, 94]; a number of patients with focal epilepsy obtained Engel Class I outcome even without resecting such regions [85, 86]. Moreover, individuals without any history of epileptic seizure can intermittently generate HFOs at >250 Hz [78]. Therefore, it is likely that some but not all sites showing interictal HFOs are causally associated with seizure generation (Table 7). During the presurgical evaluation of each patient, the significance of interictal HFOs should be assessed, by taking into account the eloquent cortex, seizure-onset zone, and cortical lesions on imaging. It is currently not recommended to blindly resect cortical sites spontaneously generating HFOs since such blind resection may unnecessarily involve eloquent cortex. It is difficult to distinguish pathological from physiological HFOs simply based on spectral frequency measures alone [91].
Figure 6. Interictal and ictal high-frequency oscillations (HFOs).
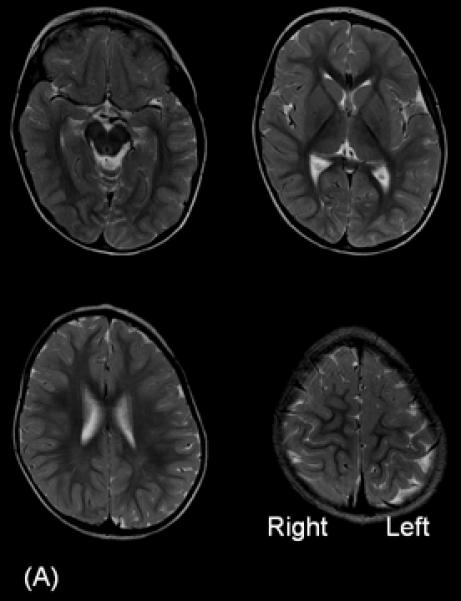
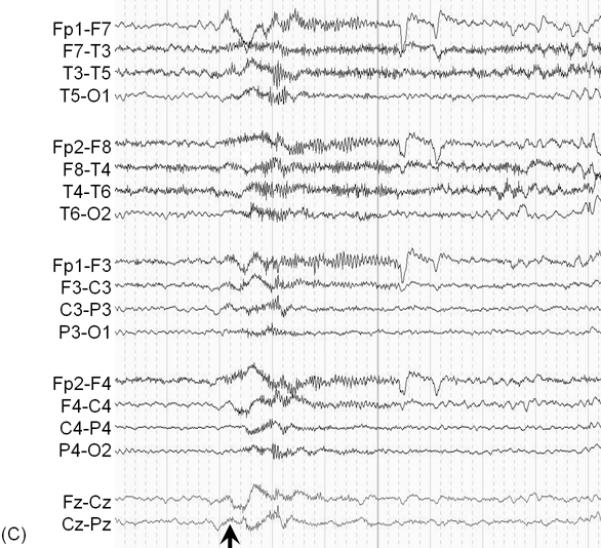
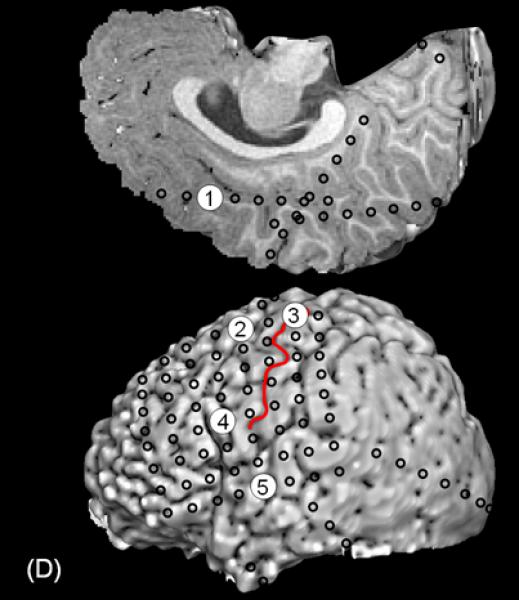
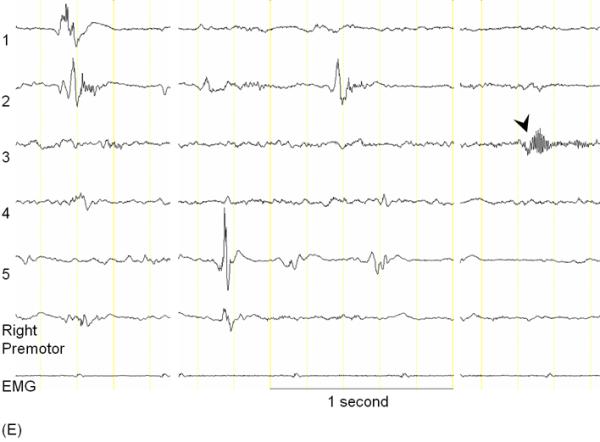
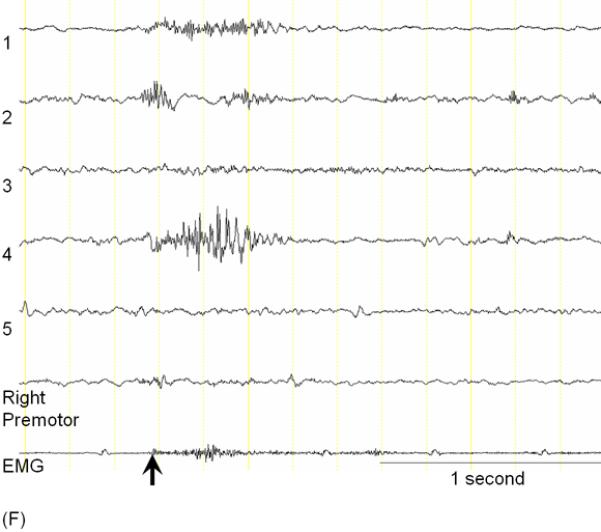
A 3-year-old, ambidextrous girl with a history of daily epileptic spasms underwent epilepsy surgery. She developed seizures at 6 months of age and had no focal neurological deficits prior to surgery. (A) Preoperative MRI showed no discrete cortical lesion except for a smaller white matter volume in the left hemisphere, especially in the frontal lobe. (B) Interictal scalp EEG is presented with a high-pass filter cuttoff of 1.0 Hz and low-pass filter cutoff of 70 Hz. The posterior background rhythm was 8 Hz alpha activity, and interictal epileptiform discharges involved the left hemisphere slightly more frequently than the right hemisphere. The left temporal lobe showed the most frequent focal spike-wave discharges. (C) Ictal EEG showed diffuse fast wave bursts superimposed on delta waves with anterior dominance; an arrow indicates the onset of a spasm. (D) Subdural grid and strip electrodes were placed on the left hemisphere. The right frontal-parietal regions were also sampled by four strip electrodes (not shown). (E) Interictal ECoG is presented with a high-pass filter cuttoff of 160 Hz and low-pass filter cutoff of 300 Hz. Interictal epileptiform discharges independently involved multiple locations in the left hemisphere such as the left frontal, temporal, parietal and occipital regions as well as the right hemisphere. The rate of interictal spike discharges in the left primary motor area for the hand (Channel 3) was much smaller than those of the remaining regions. Spontaneous high-frequency oscillations (HFOs) at >160 Hz were generated at Channel 3 during non-REM sleep (arrow). (F) Ictal ECoG during a spasm event (arrow) showed sustained and widespread HFOs involving the left premotor and supplementary motor regions earlier than the onset of epileptic spasms denoted by an arrow. Ictal HFOs involved the left hemisphere often more intensely and earlier than the right hemisphere. Following considerable discussion with the family, we decided to perform multilobar resection involving the left frontal, parietal, occipital and temporal regions, but not to remove the left Rolandic or insular cortices, in order to preserve motor function. Pathology showed gliosis without definitive dysplasia. She has been seizure-free following this surgery for four months with development of mild right hemiparesis but with maintained grasping function on the hemiparetic side. Achievement of post-operative seizure-freedom is strong evidence that the left hemispheric cortex was causally associated with the generation of epileptic spasms in this patient, and it is likely that a substantial proportion of the epileptogenic zone has been removed. Short-term follow-up data failed to support the causal relationship between HFOs spontaneously generated by the left Rolandic cortex (Channel 3) and generation of epileptic spasms, in this case. Long-term follow-up is needed to determine whether the epileptogenic zone still exists in the remaining cortical regions and whether anatomical hemispherectomy was the better option to completely eliminate seizures. It will remain unknown whether a less extensive resection would have achieved similar seizure-freedom.
Table 7.
Judgment of causality between interictal high-frequency oscillations (HFOs) and the generation of seizures.
| Criterion to judge causality | How can we conclude a causal relationship between interictal HFOs and generation of epileptic seizures? |
|---|---|
| Temporal relationship | In hippocampal slices prepared from rats, a widespread build-up of HFOs at 100–800 Hz was noted prior to the generation of seizure-like events [81, 82]. In human epilepsy, the rate of HFOs at >80 Hz can increase or decrease in the seizure onset zone as well as outside during a preictal period [76]. |
| Strength of association | The strength of association was high in two human studies [50, 80] but ranged modest to non-significant in another two human studies [77, 83]. |
| Biologic gradient (dose-response effect) | The seizure onset zone had a greater rate of interictal HFOs compared to the remaining sites [76–79]. No linear correlation was found between the size of tissue generating interictal HFOs and seizure frequency [84]. |
| Replication of findings | A significant association between the resection of HFOs and seizure outcome has been reported by studies using extraoperative and intraoperative ECoG [50, 77, 80]. |
| Coherence (consistency with other knowledge) | Eloquent cortex can generate spontaneous HFOs at >80 Hz that are physiologic in nature during sleep [85, 86]; a number of patients obtained Engel Class I outcome even without resection of the sites showing such HFOs. Somatosensory stimuli can elicit HFOs at 500–700 Hz in the post-central gyrus [88, 89]. Sensory cortices are known to spontaneously generate neural activities of which morphology is similar to that induced by a task [90]. It is difficult to distinguish pathological HFOs from physiological HFOs solely using oscillatory frequency measures [91]. The frequency of HFOs differs across regions and is generally greater in medial temporal regions compared to neocortical regions [92]. |
| Biological plausibility | Interictal HFOs can be explained by synchronized firing of abnormally bursting neurons, inhibitory field potentials, or both [72, 91]. |
| Analogy | Previous studies reported general convergence between the tissue generating interictal spikes and the seizure onset zone [52, 73–75]. Interictal HFOs often co-occur with interictal spikes [77, 92, 93]. Our prospective study of 61 patients using extraoperative ECoG reported that a lower rate of interictal epileptiform discharges in the preserved cortex was associated with a better seizure outcome, but multivariate analysis suggested that complete resection of the seizure onset zone was the only independent predictor of Engel Class I outcome [3]. |
| Specificity | HFOs are not specifically generated by the seizure onset zone. Non-epileptogenic eloquent cortex can generate spontaneous HFOs at >80 Hz of a physiologic nature during sleep [85–87]. It should be noted that eloquent cortex can potentially serve as the symptomatogenic zone (Table 1; Figure 1). |
| Experimental evidence | More complete resection of sites showing interictal HFOs resulted in better outcomes [50, 77, 80]. This experimental evidence strongly suggests that at least some sites showing interictal HFOs are causally associated with the generation of epileptic seizures. |
Significance of event-related HFOs recorded on ECoG
In the online Supplementary Document, we have discussed `the causal significance of event-related HFOs' as well as `the causal effect of pathological epileptiform discharges on such physiological HFOs'.
Conclusion
As shown in the aforementioned tables, we have discussed how to solve causality issues often raised during presurgical evaluation. One does not have to address all criteria in order to conclude a causal relationship between a finding and the outcome, but it is useful to be aware of these explicit criteria. Both neurophysiology and neuroimaging modalities complementarily contribute to a more accurate distinction of causation and association between focal findings and the generation of epileptic seizures in patients suspected of having medically uncontrolled focal epilepsy.
Supplementary Material
Acknowledgement
This work was supported by NIH grants NS47550 and NS64033. I am grateful to Harry T. Chugani, MD, Sandeep Sood, MD, Robert Rothermel, PhD, Alanna Carlson, MA, Sarah Minarik, RN, BSN, Carol Pawlak, REEG/EPT, and the staff of the Division of Electroneurodiagnostics at Children's Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A part of this work has been presented at the International Symposium on Surgery for Catastrophic Epilepsy in Infants (ISCE), the Fourteenth Annual Meeting of ISS, Tokyo, February 18–19, 2012.
REFERENCES
- 1.Lüders HO, Awad I. Conceptual considerations. In: Lüders HO, editor. Epilepsy surgery. Raven Press; New York: 1992. pp. 51–62. [Google Scholar]
- 2.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 3.Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizrahi EM. Avoiding the pitfalls of EEG interpretation in childhood epilepsy. Epilepsia. 1996;37(Suppl 1):S41–S51. doi: 10.1111/j.1528-1157.1996.tb06021.x. [DOI] [PubMed] [Google Scholar]
- 5.Benbadis SR, Lin K. Errors in EEG interpretation and misdiagnosis of epilepsy. Which EEG patterns are overread? Eur Neurol. 2008;59:267–71. doi: 10.1159/000115641. [DOI] [PubMed] [Google Scholar]
- 6.Gotman J, Levtova V, Farine B. Graphic representation of the EEG during epileptic seizures. Electroencephalogr Clin Neurophysiol. 1993;87:206–14. doi: 10.1016/0013-4694(93)90020-v. [DOI] [PubMed] [Google Scholar]
- 7.Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama T, Donner EJ, Go CY, Ochi A, Snead OC, 3rd, Rutka JT, et al. Focal-onset myoclonic seizures and secondary bilateral synchrony. Epilepsy Res. 2011;95:168–72. doi: 10.1016/j.eplepsyres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Nariai H, Nagasawa T, Juhász C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chugani HT, Shields WD, Shewmon DA, Olson DM, Phelps ME, Peacock WJ. Infantile spasms: I. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol. 1990;27:406–13. doi: 10.1002/ana.410270408. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. (18)F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002;43:1167–74. [PubMed] [Google Scholar]
- 12.Hill AB. The environment and disease: association or causation? Proc R Acad Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 13.Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76:1330–7. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 15.Smith ML, Elliott IM, Lach L. Cognitive, psychosocial, and family function one year after pediatric epilepsy surgery. Epilepsia. 2004;45:650–60. doi: 10.1111/j.0013-9580.2004.21903.x. [DOI] [PubMed] [Google Scholar]
- 16.Souza-Oliveira C, Escosi-Rosset S, Funayama SS, Terra VC, Machado HR, Sakamoto AC. Intellectual functioning in pediatric patients with epilepsy: a comparison of medically controlled, medically uncontrolled and surgically controlled children. J Pediatr (Rio J) 2010;86:377–83. doi: 10.2223/JPED.2032. [DOI] [PubMed] [Google Scholar]
- 17.D'Argenzio L, Colonnelli MC, Harrison S, Jacques TS, Harkness W, Vargha-Khadem F, et al. Cognitive outcome after extratemporal epilepsy surgery in childhood. Epilepsia. 2011;52:1966–72. doi: 10.1111/j.1528-1167.2011.03272.x. [DOI] [PubMed] [Google Scholar]
- 18.Luat AF, Asano E, Rothermel R, Sood S, Chugani HT. Psychosis as a manifestation of frontal lobe epilepsy. Epilepsy Behav. 2008;12:200–4. doi: 10.1016/j.yebeh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Sood S, Asano E, Chugani HT. Role of external ventriculostomy in the management of fever after hemispherectomy. J Neurosurg Pediatr. 2008;2:427–9. doi: 10.3171/PED.2008.2.12.427. [DOI] [PubMed] [Google Scholar]
- 20.Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010;75:2168–75. doi: 10.1212/WNL.0b013e31820203a9. [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32. doi: 10.1002/ana.20569. [DOI] [PubMed] [Google Scholar]
- 22.Huttenlocher PR, Heydemann PT. Fine structure of cortical tubers in tuberous sclerosis: a Golgi study. Ann Neurol. 1984;16:595–602. doi: 10.1002/ana.410160511. [DOI] [PubMed] [Google Scholar]
- 23.Machado-Salas JP. Abnormal dendritic patterns and aberrant spine development in Bourneville's disease--a Golgi survey. Clin Neuropathol. 1984;3:52–8. [PubMed] [Google Scholar]
- 24.Rheims S, Ryvlin P, Scherer C, Minotti L, Hoffmann D, Guenot M, et al. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia. 2008;49:2030–40. doi: 10.1111/j.1528-1167.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 25.Proserpio P, Cossu M, Francione S, Tassi L, Mai R, Didato G, et al. Insular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG study. Epilepsia. 2011;52:1781–91. doi: 10.1111/j.1528-1167.2011.03254.x. [DOI] [PubMed] [Google Scholar]
- 26.Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyllie E, Lachhwani DK, Gupta A, Chirla A, Cosmo G, Worley S, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology. 2007;69:389–97. doi: 10.1212/01.wnl.0000266386.55715.3f. [DOI] [PubMed] [Google Scholar]
- 28.Boshuisen K, van Schooneveld MM, Leijten FS, de Kort GA, van Rijen PC, Gosselaar PH, et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology. 2010;75:1623–30. doi: 10.1212/WNL.0b013e3181fb4400. [DOI] [PubMed] [Google Scholar]
- 29.Troester M, Haine-Schlagel R, Ng YT, Chapman K, Chung S, Drees C, et al. EEG and video-EEG seizure monitoring has limited utility in patients with hypothalamic hamartoma and epilepsy. Epilepsia. 2011;52:1137–43. doi: 10.1111/j.1528-1167.2011.03095.x. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Khalil B, Fakhoury T, Jennings M, Moots P, Warner J, Kessler RM. Inhibitory motor seizures: correlation with centroparietal structural and functional abnormalities. Acta Neurol Scand. 1995;91:103–8. doi: 10.1111/j.1600-0404.1995.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto R, Ikeda A, Ohara S, Kunieda T, Kimura K, Takahashi JB, et al. Nonconvulsive focal inhibitory seizure: subdural recording from motor cortex. Neurology. 2000;55:429–31. doi: 10.1212/wnl.55.3.429. [DOI] [PubMed] [Google Scholar]
- 32.Bye AM, Kok DJ, Ferenschild FT, Vles JS. Paroxysmal non-epileptic events in children: a retrospective study over a period of 10 years. J Paediatr Child Health. 2000;36:244–8. doi: 10.1046/j.1440-1754.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- 33.Kotagal P, Costa M, Wyllie E, Wolgamuth B. Paroxysmal nonepileptic events in children and adolescents. Pediatrics. 2002;110:e46. doi: 10.1542/peds.110.4.e46. [DOI] [PubMed] [Google Scholar]
- 34.Asano E, Pawlak C, Shah A, Shah J, Luat AF, Ahn-Ewing J, et al. The diagnostic value of initial video-EEG monitoring in children--review of 1000 cases. Epilepsy Res. 2005;66:129–35. doi: 10.1016/j.eplepsyres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Kutluay E, Selwa L, Minecan D, Edwards J, Beydoun A. Nonepileptic paroxysmal events in a pediatric population. Epilepsy Behav. 2010;17:272–5. doi: 10.1016/j.yebeh.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Theodore WH, Newmark ME, Sato S, Brooks R, Patronas N, De La Paz R, et al. [18F]fluorodeoxyglucose positron emission tomography in refractory complex partial seizures. Ann Neurol. 1983;14:429–37. doi: 10.1002/ana.410140406. [DOI] [PubMed] [Google Scholar]
- 37.Wong CY, Geller EB, Chen EQ, MacIntyre WJ, Morris HH, 3rd, Raja S, et al. Outcome of temporal lobe epilepsy surgery predicted by statistical parametric PET imaging. J Nucl Med. 1996;37:1094–100. [PubMed] [Google Scholar]
- 38.LoPinto-Khoury C, Sperling MR, Skidmore C, Nei M, Evans J, Sharan A, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53:342–8. doi: 10.1111/j.1528-1167.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 39.Luat AF, Asano E, Juhász C, Chandana SR, Shah A, Sood S, et al. Relationship between brain glucose metabolism positron emission tomography (PET) and electroencephalography (EEG) in children with continuous spike-and-wave activity during slow-wave sleep. J Child Neurol. 2005;20:682–90. doi: 10.1177/08830738050200081001. [DOI] [PubMed] [Google Scholar]
- 40.Chugani HT, Shewmon DA, Khanna S, Phelps ME. Interictal and postictal focal hypermetabolism on positron emission tomography. Pediatr Neurol. 1993;9:10–5. doi: 10.1016/0887-8994(93)90003-u. [DOI] [PubMed] [Google Scholar]
- 41.Nishida M, Juhász C, Sood S, Chugani HT, Asano E. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42:1275–84. doi: 10.1016/j.neuroimage.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes MD, Kutsy RL, Ojemann GA, Wilensky AJ, Ojemann LM. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin Neurophysiol. 2000;111:1802–8. doi: 10.1016/s1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh AM, Wilson SJ, Berkovic SF. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42:1288–307. doi: 10.1046/j.1528-1157.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- 44.Widdess-Walsh P, Jeha L, Nair D, Kotagal P, Bingaman W, Najm I. Subdural electrode analysis in focal cortical dysplasia: predictors of surgical outcome. Neurology. 2007;69:660–7. doi: 10.1212/01.wnl.0000267427.91987.21. [DOI] [PubMed] [Google Scholar]
- 45.Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49:758–64. doi: 10.1111/j.1528-1167.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 46.Carrette E, Vonck K, De Herdt V, Van Dycke A, El Tahry R, Meurs A, et al. Predictive factors for outcome of invasive video-EEG monitoring and subsequent resective surgery in patients with refractory epilepsy. Clin Neurol Neurosurg. 2010;112:118–26. doi: 10.1016/j.clineuro.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto A, Masuda H, Homma J, Sasagawa M, Kameyama S. False lateralization of mesial temporal lobe epilepsy by noninvasive neurophysiological examinations. Neurol Med Chir. 2006;46:518–21. doi: 10.2176/nmc.46.518. [DOI] [PubMed] [Google Scholar]
- 48.Parvizi J, Le S, Foster BL, Bourgeois B, Riviello JJ, Prenger E, et al. Gelastic epilepsy and hypothalamic hamartomas: neuroanatomical analysis of brain lesions in 100 patients. Brain. 2011;134:2960–8. doi: 10.1093/brain/awr235. [DOI] [PubMed] [Google Scholar]
- 49.Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P. Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. J Neurosurg. 2008;109:593–604. doi: 10.3171/JNS/2008/109/10/0593. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besson P, Andermann F, Dubeau F, Bernasconi A. Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain. 2008;131:3246–55. doi: 10.1093/brain/awn224. [DOI] [PubMed] [Google Scholar]
- 52.Asano E, Benedek K, Shah A, Juhász C, Shah J, Chugani DC, et al. Is intraoperative electrocorticography reliable in children with intractable neocortical epilepsy? Epilepsia. 2004;45:1091–9. doi: 10.1111/j.0013-9580.2004.65803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bollo RJ, Carlson C, Schevon C, Wisoff JH, Devinsky O, Weiner HL. Extraoperative functional mapping and staged resection of supratentorial tumors near eloquent cortex in children. Pediatr Neurosurg. 2009;45:175–80. doi: 10.1159/000218199. [DOI] [PubMed] [Google Scholar]
- 54.Onal C, Otsubo H, Araki T, Chitoku S, Ochi A, Weiss S, et al. Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. 2003;98:1017–26. doi: 10.3171/jns.2003.98.5.1017. [DOI] [PubMed] [Google Scholar]
- 55.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130:574–84. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 56.Kagawa K, Chugani DC, Asano E, Juhász C, Muzik O, Shah A, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET) J Child Neurol. 2005;20:429–38. doi: 10.1177/08830738050200050701. [DOI] [PubMed] [Google Scholar]
- 57.Carlson C, Teutonico F, Elliott RE, Moshel YA, LaJoie J, Miles D, et al. Bilateral invasive electroencephalography in patients with tuberous sclerosis complex: a path to surgery? J Neurosurg Pediatr. 2011;7:421–30. doi: 10.3171/2011.1.PEDS10348. [DOI] [PubMed] [Google Scholar]
- 58.Ochi A, Hung R, Weiss S, Widjaja E, To T, Nawa Y, et al. Lateralized interictal epileptiform discharges during rapid eye movement sleep correlate with epileptogenic hemisphere in children with intractable epilepsy secondary to tuberous sclerosis complex. Epilepsia. 2011;52:1986–94. doi: 10.1111/j.1528-1167.2011.03198.x. [DOI] [PubMed] [Google Scholar]
- 59.Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39:380–8. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 60.Park SA, Lim SR, Kim GS, Heo K, Park SC, Chang JW, et al. Ictal electrocorticographic findings related with surgical outcomes in nonlesional neocortical epilepsy. Epilepsy Res. 2002;48:199–206. doi: 10.1016/s0920-1211(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 61.Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–9. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 62.Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–8. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 63.Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–96. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 64.Khosravani H, Mehrotra N, Rigby M, Hader WJ, Pinnegar CR, Pillay N, et al. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–16. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- 65.Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122:664–71. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SC, Lee SK, Che H, Chung CK. Ictal high-gamma oscillation (60–99Hz) in intracranial electroencephalography and postoperative seizure outcome in neocortical epilepsy. Clin Neurophysiol. 2012;123:1100–10. doi: 10.1016/j.clinph.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, et al. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–97. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, et al. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45:488–96. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- 69.Ramachandrannair R, Ochi A, Imai K, Benifla M, Akiyama T, Holowka S, et al. Epileptic spasms in older pediatric patients: MEG and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Res. 2008;78:216–24. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Iwatani Y, Kagitani-Shimono K, Tominaga K, Okinaga T, Kishima H, Kato A, et al. Ictal high-frequency oscillations on scalp EEG recordings in symptomatic West syndrome. Epilepsy Res. 2012;102:60–70. doi: 10.1016/j.eplepsyres.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 71.Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci. 2011;14:635–41. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jefferys JG, de la Prida LM, Wendling F, Bragin A, Avoli M, Timofeev I, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–64. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hufnagel A, Dümpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–78. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 74.Asano E, Muzik O, Shah A, Juhász C, Chugani DC, Sood S, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44:425–34. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- 75.Marsh ED, Peltzer B, Brown MW, 3rd, Wusthoff C, Storm PB, Jr, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2010;51:592–601. doi: 10.1111/j.1528-1167.2009.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50:1780–92. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–11. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- 78.Blanco JA, Stead M, Krieger A, Stacey W, Maus D, Marsh E, et al. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain. 2011;134:2948–59. doi: 10.1093/brain/awr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, et al. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–91. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–94. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lasztóczi B, Nyitrai G, Héja L, Kardos J. Synchronization of GABAergic inputs to CA3 pyramidal cells precedes seizure-like event onset in juvenile rat hippocampal slices. J Neurophysiol. 2009;102:2538–53. doi: 10.1152/jn.91318.2008. [DOI] [PubMed] [Google Scholar]
- 82.Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haegelen C, Perucca P, Andrade-Valença L, Châtillon C, Zelmann R, Dubeau F, et al. Correlation between high-frequency oscillations, surgical outcome and extent of surgical resection in patients with medically refractory epilepsy. Epilepsy Curr. 2012;12:16. [Google Scholar]
- 84.Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Res. 2009;85:287–92. doi: 10.1016/j.eplepsyres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–17. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 86.Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33:569–83. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2012.03721.x. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- 88.Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Härer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–7. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 89.Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–18. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs J, Kobayashi K, Gotman J. High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol. 2011;122:32–42. doi: 10.1016/j.clinph.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–59. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mari F, Zelmann R, Andrade-Valenca L, Dubeau F, Gotman J. Continuous high-frequency activity in mesial temporal lobe structures. Epilepsia. 2012;53:797–806. doi: 10.1111/j.1528-1167.2012.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nariai H, Matsuzaki N, Juhász C, Nagasawa T, Sood S, Chugani HT, et al. Ictal high-frequency oscillations at 80–200 Hz coupled with delta phase in epileptic spasms. Epilepsia. 2011;52:e130–e4. doi: 10.1111/j.1528-1167.2011.03263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.