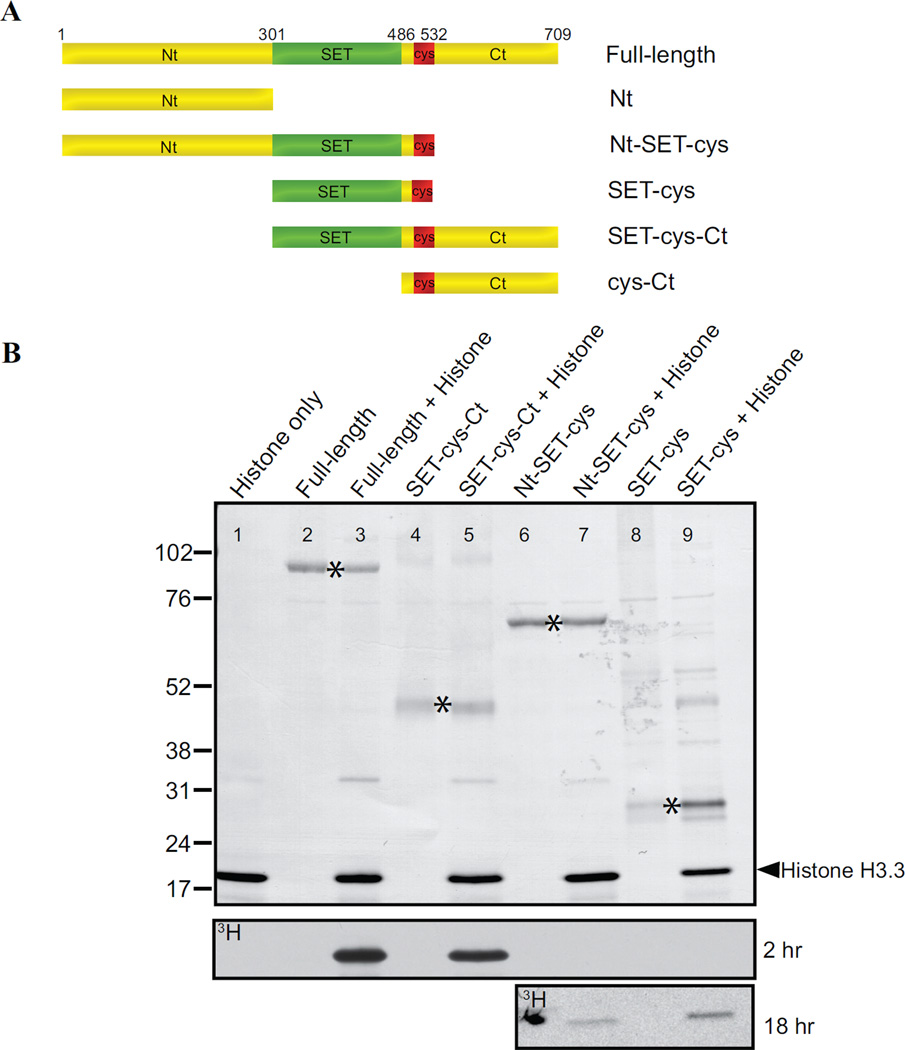
Figure 3. In vitro analyses of the lysine methyltransferase activity of AKMT truncations.
A) Schematic representation of the full-length and truncated AKMT proteins used in the study. Full-length: 2–709 aa; Nt: 2–300 aa; Nt-SET-cys: 2–532 aa; SET-cys: 301–532 aa; SET-cys-Ct: 301–709 aa; cys-Ct: 487–709 aa.
B) In vitro methyltransferase assays were performed on histone H3.3 with FLAG tagged recombinant full-length and truncated AKMT constructs that contain the putative enzymatic core, the SET-cys domain. Top: Blot stained with amido black showing total protein in the reactions: histone H3.3 alone (lane 1), full-length alone (lane 2), full-length + histone H3.3 (lane 3), SET-cys-Ct alone (lane 4), SET-cys-Ct + histone H3.3 (lane 5), Nt-SET-cys alone (lane 6), Nt-SET-cys + histone H3.3 (lane 7), SET-cys alone (lane 8), and SET-cys + histone H3.3 (lane 9). The protein truncations are indicated by an asterisk and the position of histone H3.3 is indicated by the arrowhead. Bottom: Autoradiographs showing 3H signals of the same blot after 2 hour (for all lanes) and 18 hour (for lanes 6–9) exposures. The irregularly-shaped dark spot observed (lane 6) in the 18 hr exposure panel is an artifact.