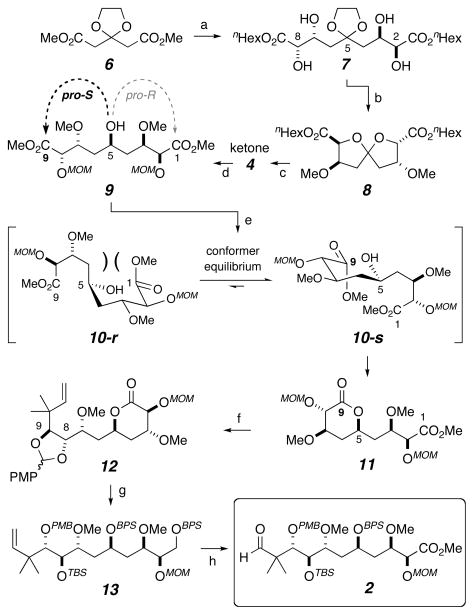
Scheme 2.
Synthesis of main fragment 2 via the key diastereoselective lactonization of pseudosymmetric carbinol 9. Reagents and conditions: (a) i) DIBAL-H, Et2O, −78 °C; then (EtO)2P(O)CH(Na)CO2nHex, 80%; ii) SAD, 0 °C, 88%; (b) i) 30 mol% HI, THF, 0 °C, 83%; ii) Me3OBF4, proton sponge, 0 °C to RT, 84%; (c) i) 1,2-ethanedithiol, BF3·OEt2, 0 °C; ii) MOMCl, i-Pr2NEt, DCM, RT, 90% over 2 steps; iii) I2, NaHCO3, acetone, H2O, 0 °C, 91%; iv) Otera’s catalyst,[22] MeOH, toluene, 90 °C, 77%; (d) Raney-Nickel, H2, EtOH, RT, 91%; (e) TMG, C6H6, RT; TFA, 98% (dr 12:1). (f) i) L-Selectride®, THF, −78 °C, 87%; ii) prenyl bromide, indium powder, DMF, 55 °C, 80%; iii) AlCl3, NaI, CH3CN, DCM, 0 °C, 92%; iv) (p-MeO)-PhCH(OMe)2, CSA, DCM, 4Å mol sieves, 87%; v) MOMCl, i-Pr2NEt, DCM, RT, 99%; (g) i) LAH, THF, 0 °C, 96%; ii) BPSCl, ImH, DMAP, DMF, RT, 92%; iii) DIBAL-H, DCM, −78 °C, 95%; iv) DMP, NaHCO3, DCM, RT; v) Zn(BH4)2,[23] DCM, 0 °C (dr 3:1), 62% (2 steps); vi) TBSOTf, 2,6-lutidine, DCM, RT, 89%; (h) i) HF·pyridine, THF, pyridine, RT, 91%; ii) DMP, NaHCO3, DCM, RT; iii) NaClO2, NaH2PO4, t-BuOH, H2O, Me2C=CHMe, RT; iv) CH2N2, RT, 87%, 3 steps; v) O3, pyridine, DCM, MeOH, −78 °C, 75%. CSA = camphorsulfonic acid; DCM = dichloromethane (CH2Cl2); DMAP = 4-dimethylamino-pyridine; DMF = dimethylformamide; DMP = Dess-Martin periodinane; dr = diastereomeric ratio; ImH = imidazole; LAH = lithium aluminum hydride; OTf = triflate; SAD = Sharpless asymmetric dihydroxylation; TFA = trifluoroacetic acid; THF = tetrahydrofuran; TMG = tetramethylguanidine.