Table 1.
Ni-catalyzed Borylation of Electron-Rich and Electron-Neutral Aryl Bromides with BBA
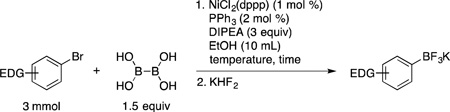 | |||||
|---|---|---|---|---|---|
| entry | product | time | temperature | yield (%) | |
| 1 | 1a | 2 h | 80 °C | 91 | |
| 2 | 1b | 2 h | 80 °C | 89 | |
| 3 | 1c | 2 h | 80 °C | 77 | |
| 4a | 1d | 6 h | rt | 78 | |
| 5a | 1e | 6 h | rt | 67 | |
| 6 | 1f | 4 h | 80 °C | 84 | |
| 7 | 1g | 3 h | 80 °C | 72 | |
| 8 | 1h | 6 h | rt | 90 | |
| 9 | 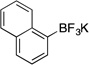 |
1i | 4 h | rt | 93 |
| 10 | 1j | 4 h 8 h |
rt | 90 81b |
|
3 mol % NiCl2(dppp) and 6 mol % PPh3
48 mmol scale using 0.1 mol % NiCl2(dppp), 0.2 mol % PPh3 in EtOH (90 mL)
