Abstract
The orbital frontal cortex (OFC) has been implicated in a number of psychiatric disorders, including depression, anxiety, phobia, and obsessive-compulsive disorder. Thus, a better understanding of its functions will likely provide critical information to understand the specific behavioral and cognitive processes affected in these human disorders. In recent years, a growing number of studies have provided evidence for anatomical and functional differentiation within the OFC. Here we discuss the effects of selective OFC (areas 11/13) lesions on social behavior, emotional regulation, and behavioral adaptation. Damage to these specific OFC subfields in adult monkeys resulted in profound changes in the flexible modulation of responses guided by reward value that could explain the poor fear regulation and disturbed social interactions observed in the same animals. A similar pattern of results was found when the OFC lesions were done in infancy. Thus, in monkeys, self-regulation abilities mediated by OFC areas 11/13 emerge from midinfancy through adolescence.
Keywords: orbitofrontal cortex, rhesus, behavior, emotion, flexibility
Introduction
Since the time of Phineas Gage,1 damage to the ventral portion of the prefrontal cortex, generally labeled orbital frontal cortex (OFC), has been associated with profoundly disturbed emotional and social behavior, as well as poor decision making in general. In humans, these behavioral and cognitive changes result from either traumatic injuries, ischemic infarcts, or surgical interventions to remove tumors or alleviate intractable epilepsy (see for review Ref. 2), and similar changes have also been reported in monkeys with OFC damage (see for review Ref. 3).
The OFC is a heterogeneous cortical area comprising several regions (Fig. 1A) that can be differentiated by their cytoarchitecture, neurochemical signature, as well as their intrinsic and extrinsic connections with other brain regions.4–7 The recent anatomical maps and connectional networks of OFC subfields4,8–10 indicate the presence of two distinct regions: an “orbital” region (areas 11/13 and insular area [IA]) and a “medial” region (areas 14/10). The orbital region is heavily interconnected with all sensory cortical areas as well as temporal lobe structures (amygdala and temporal pole area), whereas the medial region receives limited sensory inputs but reciprocates rich connections with autonomic centers. These different connectional patterns suggest different functions for the two OFC regions with the orbital region mediating the ability to determine the emotional value of events and the medial region controlling autonomic arousal.6,7
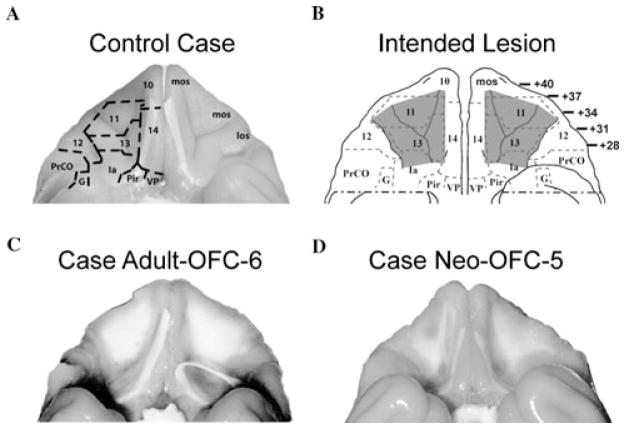
Figure 1.
Representative cases: ventral view of the macaque brain showing (A) the borders of the orbital frontal subfields on a normal brain (left) and the labels of the orbital sulci (right), (B) the intended OFC (areas 11/13) lesions shown in grey, (C) the extent of OFC damage in a representative case with adult-onset aspiration lesions (case O-asp-6), and (D) the extent of OFC damage in a representative case with neonatal-onset aspiration lesions (case Neo-O-asp-5). Abbreviations: G: gustatory cortex; Ia: insular cortex (agranular); los: lateral orbital sulcus; mos: medial orbital sulcus; Pir: piriform cortex; PrCo: precentral opercular area. Cytoarchitectonic fields are as described previously.4,91
All lesion studies investigating OFC functions in monkeys have used extensive damage to several fields, with the exception of an earlier report by Butter11 and a more recent investigation12 (see also this issue). Therefore, additional studies are needed to define the specific contribution of different OFC subfields to behavior. Accordingly, the first part of this paper summarizes experiments designed to explore the contribution of the OFC areas 11/13 (Fig. 1B) to the modulation of social behavior, reactions to stressful or potentially dangerous situations, as well as behavioral adaptation in response to changes in the value of positive affective stimuli. These studies included a total of 36 adult male rhesus monkeys (Macaca mulatta) raised under naturalistic conditions. Animals were divided into four experimental groups (n = 9 in each) that were balanced with respect to high or low social dominance rank as much as possible. One group received bilateral lesions to areas 11 and 13; three of which were created with injections of the neurotoxin ibotenic acid, whereas the remaining six cases received aspiration lesions (Fig. 1C). A second group received bilateral ibotenic acid lesions of the amygdala, including all subnuclei. A third group received ibotenic acid lesions of the hippocampal formation. The last group of sham-operated animals served as controls. Given the focus of this chapter, we will only summarize the findings from animals with the OFC lesions compared to those of the sham-operated controls.
Finally, because profound and persistent behavioral emotional and social changes have also been described in children and adolescents with ventromedial prefrontal cortex damage13–17 (Vargha-Khadem et al., 2006, personal communication), the final section of this review will survey more recent results in monkeys comparing the effects of early-onset OFC areas 11/13 lesions to those of adult-onset OFC lesions.
Social behavior assessments
To assess the impact of damage to OFC areas 11 and 13 on social behavior, animals were allowed to freely interact in groups both before and after their surgeries.18 These interactions occurred within a large indoor enclosure (3.1 m long × 1.6 m wide × 1.9–2.3 m tall) and were video recorded for in-depth analysis later. We were interested in identifying alterations in sociable or agonistic personality traits, as well as changes in the frequency, duration, or sequential exchange of social behaviors. All animals were unfamiliar when tested before surgery, and group membership remained constant when the animals were retested six months after surgery. After surgery, each group contained one animal from each of the four experimental groups. Given that social dominance status significantly dictates the type and magnitude of social behaviors initiated and received by nonhuman primates, especially male macaques,19 statistical analyses were conducted on each behavioral variable using each group either as a whole or considering social hierarchical status (dominant versus subordinate) as a contributing factor.
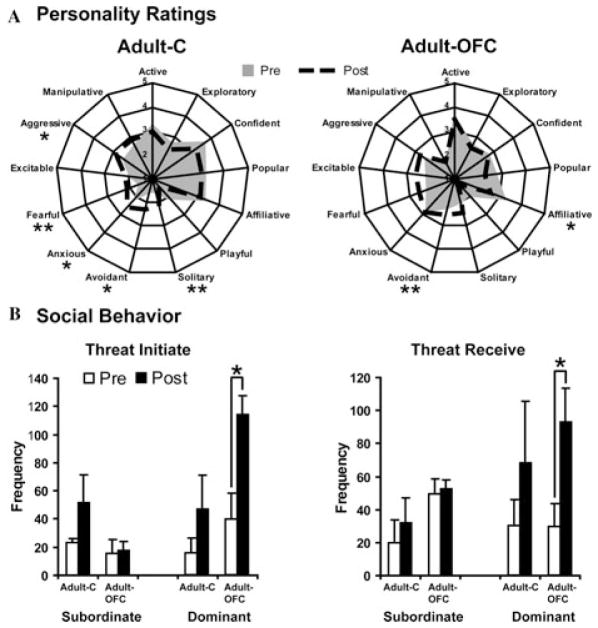
Damage to the OFC areas 11/13 yielded only mild changes in personality ratings, such as decreases in “affiliative” and increases in “avoidant” personality qualities, relative to presurgery levels (Fig. 2A, see Table 1 for personality definitions). The most interesting aspect of this finding was that the sham-operated controls demonstrated far more changes in personality traits across the two testing phases than those with OFC damage. Specifically, control animals showed increases in “solitary,” “avoidant,” “anxious,” “fearful,” and “aggressive” personality traits (Fig. 2A). These changes in personality by the control animals may have been reactions to the lesion-induced behavioral changes displayed by their social partners. Thus, the OFC may be a critical neural region for adapting general social interaction patterns, such as those measured by personality ratings, in response to changes in social context. Such personality ratings had not been used in previous studies where larger portions of the orbital frontal or prefrontal cortex were damaged. However, our interpretation of these findings was consistent with a large body of literature that had previously shown that the OFC is involved in flexibly modulating goal-directed behavior depending on changing reinforcement contingencies or outcomes (see for review Refs. 20, 21). Our own follow-up studies with these same animals also substantiated this conclusion regarding OFC function (see later).
Figure 2.
Effects of OFC lesions on social interactions for adult animals with OFC lesions (Adult-OFC) and sham-operated controls (Adult-C). In A, personality attributes are listed along the outside of the radial plots, with the shaded area representing mean scores collected before surgery (pre) and the dashed lines representing scores measured after surgery (post). All ratings were on a five-point scale, ranging from not at all descriptive (score = 1) to very descriptive (score = 5). In B, frequency of “threat initiated” and “threat received” pre- and postsurgery (white bars and black bars, respectively) are given separately for subordinate and dominant animals of each group. *P < 0.05 and * *P < 0.01 for differences between pre- and postsurgery assessments.
Table 1.
Personality categories rated for each animal before and after surgery
| Adjective | Brief definition |
|---|---|
| Active | Ambulates about the cage for the majority of the session |
| Exploratory | Readily investigates the test setting orally or manually |
| Confident | Behaves in a positive, assured manner, not restrained or tentative in any way |
| Playful | Actively and freely initiates or joins in play behavior with many partners |
| Affiliative | Sociable; seeks out the companionship of several different partners |
| Popular | The animal’s companionship is actively sought out by several different partners |
| Avoidant | Refrains from interacting with others by repeatedly exhibiting evasive behavior or physically repelling others |
| Solitary | Actively chooses to spend time alone |
| Manipulative | Tries to control the behavior of others for individual gain |
| Aggressive | Attempts to or actually causes physical harm to several other group members |
| Anxious | Tense, extremely vigilant, exhibits stereotypic behaviors |
| Excitable | Extremely reactive or overreacts to events in the group |
| Fearful | Readily fear grimaces and retreats from others; readily shows submissive postures |
Note: Each adjective listed above was rated on a five-point Likert-type scale, with definitions for each level as follows: 1 = definition not at all descriptive, 2 = definition slightly descriptive, 3 = definition moderately descriptive, 4 = definition mostly descriptive, and 5 = definition completely descriptive. All ratings were made based solely on the interactions observed on a given day. Observers were explicitly instructed to not use prior knowledge of the animals to influence how each was scored.
A second interesting change in social behavior exhibited by the animals with OFC lesions was an increase in the frequency of threatening gestures initiated to their group mates after surgery (Fig. 2B) associated with higher levels of aggression that they received from their social partners after surgery. These results were consistent with one previous report22 showing that OFC lesions largely confined to areas 11 and 13 resulted in heightened aggression. The increased aggression initiated and received by animals with OFC damage only occurred for those animals exhibiting high social dominance before and after surgery. This pattern came as little surprise considering that dominant macaque males typically initiate more threatening gestures and engage in more physical aggression than their subordinate counterparts.
Finally, to focus on how the OFC contributes to the interpretation of social signals and the production of species typical responses, a lag sequential analysis was conducted on the behavioral data collected from the pre- and postsurgical social testing sessions. More specifically, we calculated the likelihood (in terms of log odds ratio) that an animal would respond with a particular behavior within 10 sec of receiving a specific social cue. In particular, we examined how the experimental groups responded to threatening gestures and affiliative signals (e.g., a mount solicitation) they received from other partners both before and after surgery. The animals with OFC damage were unique in that they were the only experimental group to show changes in responses to both threatening and affiliative social cues. When receiving threatening gestures from animals with hippocampal lesions, dominant monkeys with OFC damage showed decreased aggressive behaviors. Control animals did not show any changes in aggressive responses to threats across conditions. In addition, animals with OFC lesions were less likely to mount when solicited by control animals, although this change was most evident for the dominant animals of the group. These results implied that OFC areas 11 and 13 are critical for the moment-to-moment interpretation of the meaning of positive and negative social cues and the modulation of adaptive behavioral responses (see later).
The confluence of findings from our social behavior assessments is largely in line with the earlier nonhuman primate study with lesions largely confined to OFC areas 11 and 13 (Ref. 22). However, the behavioral deficits were less severe than those reported following larger frontal lobe ablations that also included the OFC.23,24 In these earlier studies, monkeys with extensive prefrontal damage displayed profound decreases in positive social behaviors (grooming, huddling, near-body contact, etc.) and socially communicative facial, vocal, and postural behaviors, as well as an increase in inappropriate social interactions. These animals were ostracized from their naturalistic social groups and perished soon thereafter. Our animals with OFC damage were not ostracized within their small social groups, did not show any changes in dominance rank (relative to before surgery), and, in fact, were the most preferred social partners for control animals. However, our results provided strong evidence that areas 11 and 13 influence primate social behavior in at least two ways. First, the OFC may be critical for normal modulation of aggression, affiliation, and avoidance when a change in social context occurs or when the behavior of familiar social partners changes. Second, the OFC may flexibly represent the current value or meaning of both positive and negative social signals, thereby facilitating the selection of the most appropriate behavioral response. We followed up on these hypotheses with additional experiments that were more focused on the modulation of defensive and tension-related behaviors across several potentially dangerous conditions. We also tested how the OFC contributes to altering appetitive behavior depending on the current value of primary and secondary reinforcers. Those results are discussed in the following two sections.
Reponses to potentially dangerous situations
Since our study of OFC areas 11 and 13 function in a social context revealed changes in how those animals responded to threatening or agonistic encounters, two additional experiments were conducted with these same animals to determine whether or not these most restricted OFC lesions altered defensive or tension-related responses to several kinds of potentially dangerous stimuli. In the first study,25 we adapted a well-established paradigm that has been used extensively to assay emotional reactivity in rhesus monkeys.26–32 This so-called human intruder paradigm takes advantage of the rhesus monkey’s innate aversion to direct eye contact and their natural apprehension when unfamiliar humans enter the laboratory. In this paradigm, the animal is confronted by an unfamiliar human who either stands near the testing cage with their eye gaze averted by 90° (no eye contact or NEC condition) or stares directly at the animal (stare condition). The animals’ reactions to these conditions, both before and after surgery, were video recorded and analyzed in-depth later. The main advantage of this paradigm is that it includes both low and high threat conditions (NEC and stare, respectively) that allow to precisely measuring the contribution of OFC areas 11/13 to modulate defensive or tension-related behaviors given a change in threat magnitude.
Several important differences between sham-operated control animals and those with OFC damage were noted. First, regardless of the level of threat (NEC and stare conditions combined), control animals showed an increase in tension-related behaviors between the pre- and postsurgery testing phases. Tension-related behaviors indicate stress but are typically low in magnitude and are not necessarily directed at a stress-inducing stimulus (i.e., they are also observed when rhesus monkeys are alone). For example, monkeys displaying tension-related behaviors may yawn, scratch their torso or other body part, pace around the test cage and/or emit contact (“coo”) vocalizations. Animals with OFC damage did not show this same change in tension-related behaviors. This pattern of results is reminiscent of the negligible changes in personality traits by the same animals with OFC damage between pre- and postsurgery assessments in the social context (see above), relative to more extensive changes displayed by control animals. In addition, although all animals were able to modulate the magnitude of tension-related behaviors according to the levels of threat given by the intruder (i.e., higher tension-related behaviors during the stare condition than the NEC condition) prior to surgery, this regulation of emotional reactivity remained present after surgery for the control animals but not for those with OFC damage (Fig. 3A). This pattern of results could not be due to deficient visual attention in the operated groups given that the amount of time spent looking toward the human intruder did not vary between groups both pre- and postsurgery. Therefore, these results again reinforced the idea that damage to areas 11 and 13 results in an inability to adapt behaviors related to stress or danger depending on the particular situation, and were not reported in other similar investigations of monkeys with more extended OFC damage.31,32
Figure 3.
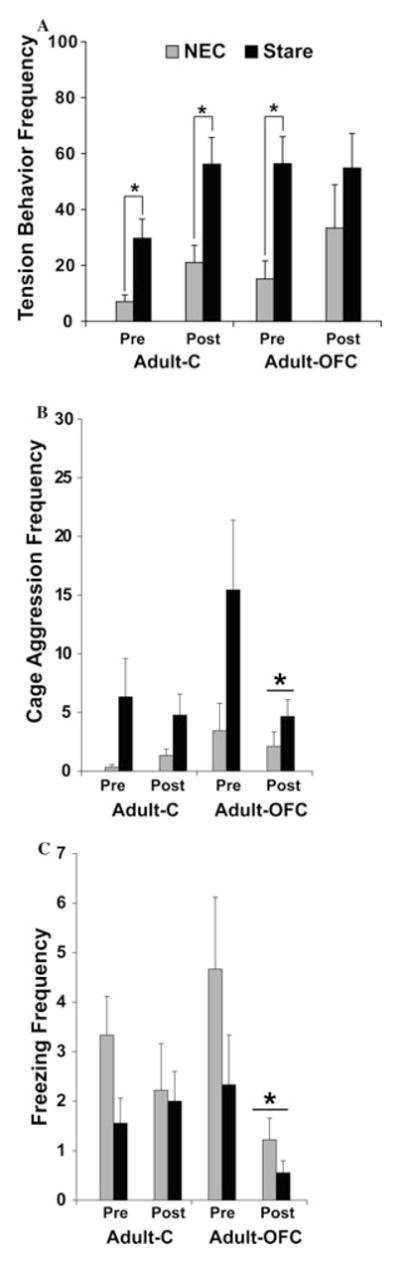
The total frequency of tension-related behaviors (A), cage aggression (B), and freezing (C) during the no eye contact (NEC) or stare conditions of the human intruder paradigm for sham-operated controls (Adult-C) or animals with OFC lesions (Adult-OFC). Data are shown for both groups both before (pre) and after (post) surgery. *P < 0.05, difference between NEC and stare conditions in each testing phase.
Beyond generalized tension-related behaviors, animals with OFC areas 11/13 damage also demonstrated diminished defensive behaviors in the human intruder paradigm. Defensive behaviors are more overt and higher in magnitude than tension-related behaviors, and are typically only displayed when the stress-inducing stimulus (i.e., the unfamiliar human) is physically present. Regardless of condition (NEC and stare combined), OFC damage resulted in significant decreases in three defensive behaviors after surgery, namely cage-shaking (Fig. 3B), tooth-grinding, and freezing (Fig. 3C), which were not observed in the control animals. Diminished freezing following OFC damage (also largely confined to areas 11 and 13) has been reported in a separate study by Kalin et al.,32 but not when lesions also included area 14).31 A pattern of diminished defensive behaviors following lesions more restricted to areas 11 and 13 has not been reported previously. On the contrary, when the OFC lesions included also area 14, an increase in mild aggression has been reported.31
In our second study of threat-induced behavioral reactivity,33 we again adapted a well-established paradigm that focused on the monkeys’ willingness to approach neutral or potentially dangerous objects. 27,28,31,32,34–38 Although previous renditions of this paradigm typically used a very limited number of aversive stimuli (typically real or fake snakes and size-matched neutral objects), we greatly expanded the number and type of aversive objects to investigate the role of the OFC areas 11/13 in approach-avoidance conflict for stimuli representing potential predators (e.g., coiled rubber snake), and also those that the monkeys had learned to fear before surgery by living in a laboratory environment (e.g., capture net, leather handling gloves, a hypodermic syringe, etc.). Each of these aversive objects had a size- and shape-matched “neutral” object that served as a control stimulus. This particular experiment was only conducted after surgery, since experience with these inanimate objects during a presurgery phase would have greatly diminished their aversive nature in any subsequent postsurgery testing sessions. Each neutral or aversive object was only shown once and was placed adjacent to a highly preferred food (a green grape) on a testing tray. The animal was allowed 60 sec to visually or manually explore the object and retrieve the food. All behavior was video recorded for in-depth analysis later.
In contrast to the human intruder paradigm, we found no differences between monkeys with OFC damage and controls in the occurrence of defensive or tension-related behaviors, as well as the latency to retrieve the preferred food reward for any of the neutral or aversive inanimate objects. Therefore, initial reactivity and avoidance of potentially dangerous items was not altered by selective lesions of areas 11 and 13. This would appear to be in contrast to several previous reports of diminished avoidance following OFC lesions using this paradigm.31,32 However, in those previous studies, animals were exposed to the aversive objects (primarily a rubber snake) on multiple test days. The differences in avoidance between control and experimental groups did not emerge until after several exposures; initial avoidance was not different from control animals. Therefore, the core deficit for the animals with OFC lesions could be an inability to extinguish or maintain an adaptive behavioral pattern in a given context (e.g., presence of a predator or loss of reinforcement where it was previously experienced). Support for this idea already exists since impairment in extinction of instrumental responses emerges after extended damage to areas 11, 13, and 14 or damage restricted to area 14, but not after damage restricted to areas 11/13 (Refs. 12, 31).
The picture that is emerging from these more focused experiments of how the OFC influences behavior when animals are exposed to potentially dangerous stimuli is quite complex and certainly warrants more attention. One interpretation of our findings, as well as those of others that have recently studied the effects of circumscribed OFC lesions39,40 is that this cortical region is not necessarily required for normal avoidance or wariness of potentially dangerous stimuli. That seems to be one function of the amygdala. However, areas 11 and 13 do appear to be necessary for normal flexible modulation of tension-related behaviors depending on the magnitude of threat.
Changing behavior according to reinforcement contingencies
As mentioned above, our investigation of OFC functions in the social context revealed that damage to areas 11 and 13 altered how these animals reacted to positive social signals. To follow-up on this result in more controlled experiments, we chose to study these same animals in three experiments that were based on the selection of foods, as well as the modulation of these choices depending on changes on the reward value of the stimuli41 or on changes in the internal motivational state of the animal.42
Object reversal learning
In earlier studies, the object reversal task has been instrumental for the demonstration of an important contribution of the OFC in behavioral adaptation in response to changes in reward contingency of positive stimuli. Damage to the OFC results in reversal learning deficits in several species,11,43–58 such that this task has become one benchmark for assessing functionality of the OFC. For monkeys, the reversal deficit usually follows damage that encompasses several OFC subregions, including the middle areas 11/13, rostral area 10, ventromedial areas 14/25, and, in some instances, lateral areas 12/47 (Refs. 44, 48, 57, 59). However, Butter11 already demonstrated that not all OFC subfields are critical for object reversal performance (see also Refs. 60 and 61). Thus, following testing of social behavior and emotional reactivity, we tested the animals in the object reversal task (i.e., approximately eight months after surgery). In the reversal task, the animals first learned which of the two objects in a pair was consistently rewarded from trial to trial. After mastering the task, the reward contingency of the two objects was reversed, such that the rewarded object became unrewarded and vice versa. Six such reversals were given in succession. Animals with selective lesions to OFC areas 11/13 performed as well as controls (see Fig. 4B, groups Adult-C and Adult-OFC), although the total number of reversal errors committed by animals with OFC lesions correlated with additional damage to area 14. Although these results suggested that OFC area 14 may be more critical than areas 11/13 for reversal learning, selective lesions to area 14 has recently been shown to have no effects on reversal learning.12 Together with the severe deficits in reversal learning after large OFC lesions,48,57 the newer findings suggest that these deficits were the result of either combined damage to both the orbital and medial OFC regions (areas 11, 13, and 14) or damage to fibers connecting the OFC regions with lateral prefrontal area 12 (see also Ref. 11).
Figure 4.
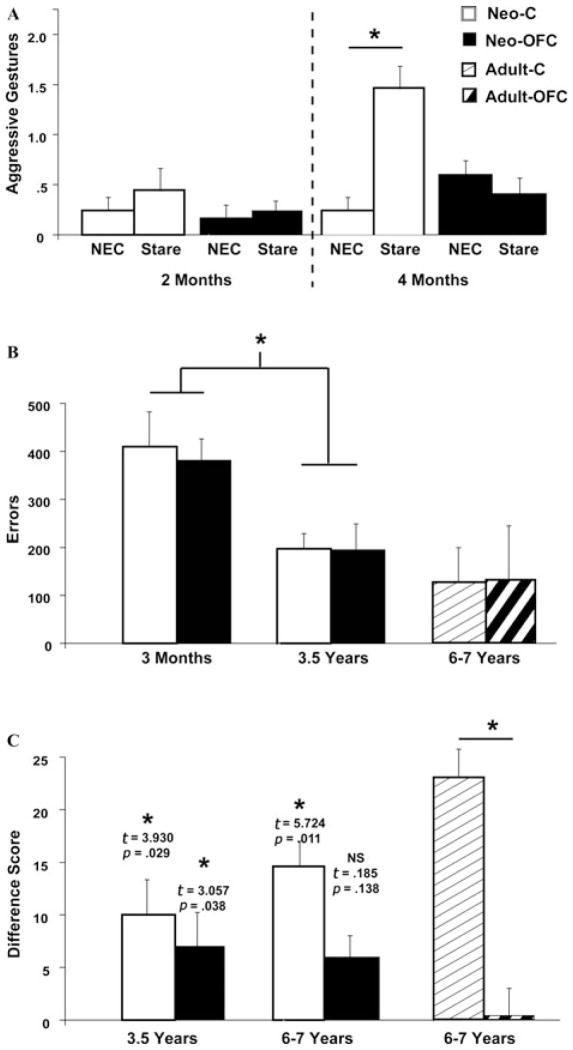
Effects of neonatal OFC lesions on emotional reactivity toward the human intruder (A), reversal learning (B), and reinforcer devaluation (C). For each task, white bars represent animals with neonatal sham-operations (Neo-C), black bars represent animals with neonatal OFC lesions (Neo-OFC), bars with thin stripes represent animals with adult sham-operations (Adult-C), and bars with thick stripes represent animals with adult OFC lesions (Adult-OFC). *P < 0.05 indicates significant differences between the NEC and stare conditions in A, significant age differences for both groups in B, and significant differences from chance in C.
Food preference and devaluation of primary reinforcers
In the second experiment, simple preferences for foods (raisins, peanuts, candies, etc.) and nonfoods (paper balls, pieces of cork, etc.) were examined both before and after surgery. On each trial, two different foods, two different nonfoods, or one food and one nonfood were presented to the animal on a testing tray. The animal was allowed 15 sec to select one, both or neither item, and selection priority was recorded. In contrast to several earlier accounts of larger OFC damage,39,62,63 damage restricted to areas 11 and 13 did not alter any presurgical preferences for the food or nonfoods used in this study.
We then measured how a change in physiological or motivational state would affect their preference for a favorite food versus other less-preferred foods.64 We reduced the animal’s motivation for their favorite food by satiating the animals on that food prior to food preference testing, as had been done by others.36,65–70 Following satiation, animals were presented with 30 pairs of food and nonfood items. Each pair contained the devalued (sated) food along with one other food or nonfood. The difference in preference for their favorite food between the postsurgery testing phase (see above) and following devaluation by satiation (i.e., difference score = preference when not sated—preference when sated) was used to measure the ability to flexibly change food selection. Control animals showed a robust, positive difference score (62%) since their preference for their favorite food was greatly diminished following satiation. By contrast, animals with ibotenic acid OFC lesions (including areas 11 and 13, as well as portions of areas 12, 14, and the agranular IA) showed a significantly lower difference score (25%), indicating that they did not switch their preference for the devalued food as much as controls after satiation. Animals with aspiration lesions of the OFC that were more restricted to areas 11 and 13 showed an intermediate difference score (40%) that was not different from controls or those animals with the larger ibotenic acid OFC lesions.
Devaluation of conditioned reinforcer
In a third study, we examined the role of the OFC in interpreting the meaning of conditioned reinforcers. Social signals are conditioned reinforcers; their meaning must be learned through experience and meaning must also be updated by new experiences or changes in emotional or motivational state. To study this aspect of OFC function, we again used a reinforcer devaluation strategy71 to test whether monkeys with selective damage to OFC areas 11/13, which demonstrated striking sparing in adapting their responses to changes in reward contingency (see above), would also be able to adapt their responses to changes in the reward value of stimuli. In this case, the animals were first required to learn the reinforcement contingencies and values of 60 pairs of objects. In each pair, one of the two objects covered one of two food rewards, either a raisin (30 objects) or a peanut (30 objects). The 60 pairs were presented one at a time, with the raisin pairs and the peanut pairs intermixed within a daily session, and occurred in the same order from one day to the next. The animals needed to learn to displace the items covering rewards on 90% or more trials to complete this acquisition phase. There was no difference between controls and animals with lesions of areas 11 and 13 in the total number of errors or the total number of testing days to reach the learning criterion.
After this learning, a reinforcer devaluation phase, which included four sessions, was introduced. In this phase, the unrewarded objects of the 60 pairs were discarded and a “raisin” object was paired with a “peanut” object to form 30 pairs of discrimination. In two control sessions, the 30 pairs were presented and animals selected one of the two rewarded objects. In two other critical sessions, the animals were satiated with either raisins or peanuts before being presented with the 30 pairs. The difference between preference for “raisin-associated objects” and “peanut-associated objects” when the animals were not satiated and when they were satiated was calculated. Control animals again showed robust, positive difference scores, indicating that they were able to update the value of each object following satiation (see Fig. 4C, group Adult-C). By contrast, animals with aspiration OFC lesions confined to areas 11 and 13 showed significantly lower difference scores, indicating that they continued to select items associated with the sated food (see Fig. 4C, group Adult-OFC). These results are similar to other previous studies of monkeys with OFC damage including areas 11, 13, and 14,68 as well as those with crossed disconnection of the OFC and amygdala.67 The experiments thus far converge on the idea that OFC areas 11 and 13 are critical for guiding goal-directed behavior (both social and nonsocial) with regard to changes in emotional or motivational state (see also Ref. 12).
Together, the present findings suggest that OFC areas 11/13 are necessary to adjust choices based on reward value but less so for choices based on reward contingency. This latter function could perhaps involve other OFC fields, including the most medial subgenual fields, area 25, and the most lateral, area 12.7,72 Future studies are clearly required to better identify the specific interactive processes by which different OFC fields support reversal learning.
Conclusions concerning the role of OFC areas 11/13 in behavioral adaptation
Selective damage to OFC areas 11/13 yielded (1) significant changes in social behavior, characterized by an inability to interpret the meaning of positive and negative social cues, and to modulate adaptive behavioral responses during active social interactions; (2) deficits in normal modulation of tension-related behaviors relative to the magnitude of threat and in maintaining a pattern of avoidance or fear over time or across separate exposures to a threat; and (3) inability to flexibly adjust choice selection based on reward value, while sparing the ability to adjust choice selection based on reward contingency. One of the main goals of our study was to assess whether the profound deficit in behavioral adaptation found after selective OFC area 11/13 lesions correlated with their inability to regulate emotional reactivity and behavioral responses to social cues.
It is interesting to note that the reinforcer devaluation deficit after damage to OFC areas 11/13 was associated with difficulty in modulating emotional reactivity when animals with OFC lesions were challenged with social stimuli differing in the magnitude of threat as assessed by the human intruder task.73 Before surgery, all animals showed higher frequency of tension-related behaviors in the stare condition than in the NEC condition, indicating that they could modulate their emotional responses according to the intensity of threat provided by the intruder. After surgery, however, only sham-operated controls continued to demonstrate this ability. Thus, as for the reinforcer devaluation tasks, OFC areas 11/13 seem critical to flexibly adjust emotional reactivity based on the magnitude of negative signals, such as threat. Similarly, the impairment in monitoring the positive and negative value of stimuli after OFC areas 11/13 lesions could also be the source of the striking behavioral changes found in animals with OFC lesions when interacting in small familiar social groups.74 In this latter study, we reported that animals with OFC areas 11/13 lesions were involved in more aggressive interactions and responded abnormally to both affiliative and threatening signals. All together the data demonstrate that flexible decision-making mechanisms mediated by OFC areas 11/13 are critical to support normal social behavior. Further studies will be required to similarly assess the role of other OFC subfields in behavioral adaption that mediates emotional regulation and social behavior.
Developmental outcomes of early-onset damage to OFC areas 11/13
Although a majority of the developmental literature has focused on executive functioning (problem solving, abstract reasoning, etc.) mediated by the dorsolateral prefrontal cortex, research on the development of OFC functions has lagged behind. Yet, the OFC has been implicated in several developmental neuropsychiatric disorders associated with emotional and social abnormalities, such as Autism and William’s syndrome.75–79 Therefore, a better understanding of how OFC maturation is linked to socioemotional development is urgently needed to facilitate development of novel treatments for these disorders.
Infants are born into complex social groups and are faced with the developmental task of coming to respond differentially and appropriately to many categories of social partners as well as to individuals within those categories. Thus, during development, individuals progressively learn complex rules for self-regulation of emotion and behavioral adaptation that assure successful social relationships. Hence, the OFC is likely to be a critical player in the normal development of these cognitive skills. The OFC is known to have a protracted development.80–82 Age-related changes in both humans and monkeys have also been reported on tasks measuring behavioral adaptation (e.g., object discrimination reversal, extinction, and Iowa gambling tasks) that have been associated with OFC damage in adulthood (see for review Refs. 83 and 84).
The earlier work of Goldman-Rakic et al. has demonstrated that extensive early-onset damage to the frontal cortex, which included the OFC, yielded progressive impairment in reversal learning, as well as reduced level of play behavior and increased aggressive gestures (see for review Ref. 80). Few reports in humans13–16 (Vargha-Khadem et al., 2006, personal communication) also indicate that ventromedial prefrontal damage acquired in childhood places individuals at risk for failure to develop normal social or occupational competencies in adolescence and adulthood. These behavioral and social changes reflect chronic emotional disruption and impairments of decision making, planning, and behavioral regulation. The long-term impairments associated with childhood-onset lesions are at least as severe as those resulting from adult-onset damage. In addition, these impairments, which persist for decades following childhood ventromedial injuries, stand in sharp contrast to the relatively good functional recovery found after childhood damage to other brain regions, such as the relatively normal development of language following early damage to the left perisylvian region.
Thus, a growing interest in the anatomical and functional heterogeneity of the OFC incited a consideration of its role in emotional and social development throughout life. Given the functions of OFC areas 11/13 in adult monkeys described in the first part of this paper, we began investigating the developmental outcomes of damage to OFC areas 11/13 on emotional reactivity and behavioral adaptation in infant monkeys. As for the adult-onset lesions, we prepared infant rhesus monkeys with either selective lesions of OFC areas 11/13 (n = 6; Fig. 1D) or sham operations (n = 6), which were performed when the animals were 8–10 days of age. The two groups included three males and three females. Using the same tasks as those used for the adult monkeys, we measured the behavioral effects of neonatal OFC lesions on emotional regulation and flexible response selection at different time points during maturation. A summary of the results that are currently being published is provided below.
Reponses to potentially dangerous situations
Between four and six months of age, monkeys can adaptively modulate their defensive reactions to meet changing environmental demands.85,86 At the same age, infant monkeys respond with different emotions to specific facial expressions, and display fear of strangers,87 suggesting that the brain circuits needed to discriminate threatening cues are mature. Thus, we investigated the role of the OFC areas 11/13 in the development of defensive behaviors toward an unfamiliar human (human intruder paradigm, see previous task details) both before and after this developmental critical period (i.e., at two and six months of age) and retested the animals in adulthood to assess whether any deficit will be transitory or permanent. Two-month-old monkeys, operated and control alike, showed increased frequency of defensive reactions to the presence of the intruder, but none modified their level of defensive reactions according to the magnitude of threat presented by the Intruder (e.g., “NEC” vs. “stare” conditions, see Fig. 4A). At six months of age, however, sham-operated animals did modulate the amount of emotional reactivity between the two conditions, showing greater amount of freezing in the NEC relative to the stare conditions and also more aggressive gestures in the stare than in the NEC conditions (Fig. 4A). Therefore, by the age of six months, infant monkeys can modulate their emotional responses to different threat intensities, like adult animals (see Fig. 3 for comparison), a finding similar to that reported by Kalin et al.86 By contrast, such modulation was not apparent in the neonatally OFC-operated monkeys (Fig. 4A). However, the neo-OFC infants were not totally hypoemotional since they showed an overall increase in their emotional reactivity in both conditions compared to when they were alone. The same pattern of changes in emotional reactivity was still present when these same animals were retested in the intruder paradigm (using a new human intruder) when they reached seven years of age, indicating no recovery over time.88,89 These data indicated that OFC areas 11/13 are critical for the development of fear modulation toward threatening social stimuli after the age of two months.
We hypothesized that, as for the adult-onset OFC lesions, the lack of fear modulation following neonatal OFC lesions could have resulted from an inability to use the valence of stimuli (less or more threatening) to modulate behavioral responses. To examine this possibility, we tested the ability of the same animals to flexibly modify their choice selection when the reward contingency (reversal learning) or the valence (reinforcer devaluation) of stimuli was changed.
Changing behavior according to reinforcement contingencies
Object reversal learning
Earlier studies (see for review Ref. 80) have reported a significant sex difference in the development of reversal learning following neonatal OFC lesions. Male and female infant rhesus monkeys received either extensive OFC lesions, which encompassed several OFC subfields, at 2.5 months of age or remained as unoperated controls. They were tested on reversal learning at different ages beginning at 2.5 months. Normal males outperformed normal females at 2.5 months but not at 15 months, indicating that this ability matured earlier in males than in females. In addition, whereas male infants with OFC lesions showed clear deficits in reversal learning as early as 2.5 months, the females with the same lesions did not show deficits until after their first year. Thus, the failure to observe a deficit in the OFC-operated infant females prior to 15 months of age suggests that the OFC of female monkeys does not mature until that age. By contrast, the presence of impairment in males as early as 2.5 months indicates that the OFC of male monkeys matures very early. Given that damage to OFC areas 11/13 in adult monkeys does not affect reversal learning,12,90 we investigated whether a similar outcome will follow neonatal OFC lesions restricted to areas 11/13. Given the earlier timing of our lesions (8–10 days old vs. 2.5 months for the studies of Goldman-Rakic et al.80), we also investigated whether earlier damage to OFC areas 11/13 might yield more pervasive deficits in reversal learning. Thus, animals with neonatal OFC area 11/13 lesions and their sham-operated controls were tested in discrimination reversal learning (see previous task details) at three months of age and retested at three years of age.90
At three months of age, male and female infants of both groups made significantly more errors than adult monkeys (five to six years, see Fig. 4B). However, animals of both groups improved their performance to an adult level of proficiency when retested at three years. Thus, the ability to flexibly alter response selections guided by reward contingency is not fully functional at three months of age in both male and female monkeys. Our data did not replicate the sex difference reported in the earlier studies,80 even when males and females of both groups were combined (Total reversal errors: 383.50 ± 25.97 and 416.67 ± 34.47 for males and females, respectively; t(10) = 0.768, not significant). However, this is not too surprising given the small number of animals used in our study and given that the OFC lesions were small and restricted to areas 11/13. The data indicate (1) that the neural structures mediating reversal learning abilities have a protracted development, but (2) that OFC areas 11/13 are not critical for the development of these abilities and this is true both during development and in adulthood.
Reinforcer devaluation
Given that adult-onset lesions of OFC areas 11/13 spared reversal learning but severely impaired the ability to switch response selection when the reward value of stimuli changes,12,64 we assessed whether the same pattern of results will follow early-onset lesion of OFC areas 11/13. Thus, animals with the neonatal OFC lesions and their controls were tested in the reinforcer devaluation task (see previous task description) when they were 3.5 years of age and retested on the same task at six to seven years using the same 60 pairs of stimuli. First, the ability to concurrently learn large stimulus sets (60 pairs) was mildly affected by neonatal OFC damage when animals were tested at 3.5 years old. Neonatal-operated OFC animals required more days to reach learning criterion (21 days) than their controls (13 days), but this mild deficit in stimulus–reward association did not persist when they were retested at six to seven years. Second, when the objects were devalued via the reinforcer devaluation paradigm, sham-operated controls obtained difference scores that were significantly different from chance at both ages (Fig. 4C). These results suggest that the ability to flexibly modulate response selection when the reward value of stimuli has been modified is already present in late adolescence (3.5 years) in monkeys. By contrast, at both ages, animals with neonatal OFC damage failed to use internal satiety signals to flexibly shift their response selection away from the devalued objects and obtained difference scores that were not significantly different from chance. Although at 3.5 years the group effect did not reach significance, at six to seven years of age the group effect was nearly significant for the neonatal lesions, t(7) = 1.975, P = 0.09. Thus, the failure to regulate response selection after lesions to OFC areas 11/13 is present whether the damage occurs in infancy or adulthood and is associated with a dysregulation of emotional reactivity (see above).
Summary of developmental outcomes of neonatal damage to OFC areas 11/13
The data on the sham-operated controls are interesting because they suggest that the ability to self-regulate behavioral responses progressively emerges from midinfancy through adolescence. The ability to regulate fear-related responses toward threatening signals of different intensity emerges between two and four months of age. The ability to modulate response strategy to positive stimuli when the reward contingency changes is also not present by three months of age but reaches an adult level of proficiency by three years. Finally, the ability to modulate response strategy to positive stimuli when the reward value has changed is present at 3.5 years. However, future studies are needed to more specifically assess the exact age at which the regulation of choices guided by both reward contingency (object reversal) and reward value (reinforce devaluation) emerges.
Similar to adult-onset OFC lesions, selective neonatal lesions of OFC areas 11/13 yielded a failure to modulate fear-related responses and behavioral responses guided by reward value. Thus, overall our data demonstrate little sparing of OFC functions, if any, after neonatal damage to these cortical areas. We suggest that OFC areas 11/13 play a critical role in the development of behavioral adaptation; an ability essential for the self-regulation of emotion and behavior that assures the maintenance of successful social relationships.
We realize that our developmental studies represent only a first step toward an understanding of the development of orbital frontal functions in primates. Further research is required to assess the precise time point in development at which these OFC functions emerge. It will also be important to determine if the development of behavioral regulation in response to negative affective cues is similar to that of positive affective cues, and which OFC subfields mediate such abilities.
Acknowledgments
This work has been supported by RO1-HD 35471; RO1-MH 58846; Yerkes Base Grant NIH RR00165; Center for Behavioral Neuroscience Grant NSF IBN-9876754; and an Autism Speaks Mentored-based Fellowship to JB and F32-MH63577 to CM.
Footnotes
Conflicts of interest
The authors declare no conflicts of interests
References
- 1.Harlow J. Recovery after passage of an iron bar through the head. Publ Massachu Med Soc. 1868;2:329–346. [Google Scholar]
- 2.Zald DH. Neuropsychological assessment of the orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; New York, NY: 2006. pp. 449–480. [Google Scholar]
- 3.Bachevalier J, Meunier M. Neurobiology of Socio-Emotional Cognition in Primates. Psychology Press; Hove, UK: 2005. [Google Scholar]
- 4.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 5.Robbins TW, et al. Neurochemical modulation of orbitofrontal cortex function. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; New York: 2006. pp. 393–422. [Google Scholar]
- 6.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 7.Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 9.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 10.Barbas H, et al. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- 11.Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- 12.Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SW, et al. Impairment os social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SW, et al. The earliest behavioral expression of focal damage to human prefrontal cortex. Cortex. 2007;43:806–816. doi: 10.1016/s0010-9452(08)70508-2. [DOI] [PubMed] [Google Scholar]
- 15.Anderson SW, et al. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- 16.Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain Cogn. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 17.Eslinger PJ, et al. Developmental consequences of childhood frontal lobe damage. Arch, Neurol. 1992;49:764–769. doi: 10.1001/archneur.1992.00530310112021. [DOI] [PubMed] [Google Scholar]
- 18.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 19.Cheney DL, Seyfarth RM. How Monkeys See the World. The University of Chicago Press; Chicago: 1990. [Google Scholar]
- 20.Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenbaum G, et al. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Wars) 1972;32:525–565. [PubMed] [Google Scholar]
- 23.Franzen EA, Myers RE. Neural control of social behavior: prefrontal and anterior temporal cortex. Neuropsychologia. 1973;11:141–157. doi: 10.1016/0028-3932(73)90002-x. [DOI] [PubMed] [Google Scholar]
- 24.Myers RE, Swett C, Miller M. Loss of social group affinity following prefrontal lesions in free-ranging macaques. Brain Res. 1973;64:257–269. doi: 10.1016/0006-8993(73)90182-0. [DOI] [PubMed] [Google Scholar]
- 25.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to danger: Effects of selective amygdala, hippocampal or orbital frontal lesions. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 27.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalin NH, et al. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- 30.Mason WA, et al. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatr. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 35.Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biol Psychiatr. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- 37.Mineka S, Keir R, Price V. Fear of snakes in wild-and laboratory-reared rhesus monkeys (Macaca mulatta) Anim Learn Behav. 1980;8:653–663. [Google Scholar]
- 38.Rudebeck PH, et al. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 39.Butter CM, McDonald JA, Snyder DR. Orality, preference behavior, and reinforcement value of nonfood object in monkeys with orbital frontal lesions. Science. 1969;164:1306–1307. doi: 10.1126/science.164.3885.1306. [DOI] [PubMed] [Google Scholar]
- 40.Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Warsz) 1972;32:525–565. [PubMed] [Google Scholar]
- 41.Kazama A, Bachevalier J. Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys’ performance on the object discrimination reversal task. J Neurosci. 2009;29:2794–2804. doi: 10.1523/JNEUROSCI.4655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- 43.Teitelbaum H. A comparison of effects of orbitofrontal and hippocampal lesions upon discrimination learning and reversal in the cat. Exp Neurol. 1964;9:452–462. doi: 10.1016/0014-4886(64)90053-6. [DOI] [PubMed] [Google Scholar]
- 44.Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 45.Rolls ET, et al. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatr. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 47.Bechara A, et al. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 48.Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 49.Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal–thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- 50.Schoenbaum G, et al. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 51.Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behaviour in rats under reversal conditions. Behav Brain Res. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 52.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 54.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Schoenbaum G, et al. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hornak J, et al. Reward-related reversal learning after surgical excisions in orbitofrontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 57.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior pre-frontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 60.Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 62.Ursin H, Rosvold HE, Vest B. Food preference in brain lesioned monkeys. Physiol Behav. 1969;4:609–612. [Google Scholar]
- 63.Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Exp Brain Res. 1991;86:617–622. doi: 10.1007/BF00230535. [DOI] [PubMed] [Google Scholar]
- 64.Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- 65.Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornton JA, Malkova L, Murray EA. Rhinal cortex ablations fail to disrupt reinforcer devaluation effects in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1998;112:1020–1025. doi: 10.1037//0735-7044.112.4.1020. [DOI] [PubMed] [Google Scholar]
- 67.Baxter MG, et al. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 75.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Hardan AY, et al. Magnetic resonance imaging study of the orbitofrontal cortex in autism. J Child Neurol. 2006;21:866–871. doi: 10.1177/08830738060210100701. [DOI] [PubMed] [Google Scholar]
- 77.Loveland KA, et al. Fronto-limbic functioning in children and adolescents with and without autism. Neuropsychologia. 2008;46:49–62. doi: 10.1016/j.neuropsychologia.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loveland KA, et al. Judgments of auditory-visual affective congruence in adolescents with and without autism: a pilot study of a new task using fMRI. Percept Mot Skills. 2008;107:557–575. doi: 10.2466/pms.107.2.557-575. [DOI] [PubMed] [Google Scholar]
- 79.Mimura M, et al. A preliminary study of orbitofrontal activation and hypersociability in Williams syndrome. J Neurodevelop Disord. 2010;2:93–98. doi: 10.1007/s11689-009-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman-Rakic PS, et al. Handbook of Child Psychology: Biology and Infancy Development. Wiley; New York: 1983. The neurobiology of cognitive development; pp. 282–344. [Google Scholar]
- 81.Goldman-Rakic PS, Bourgeois J, Rakic P. Development of the Prefrontal Cortex. Brooks Publishing Company; Baltimore, MD: 1997. Synaptic substrate of cognitive development; pp. 27–47. [Google Scholar]
- 82.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 83.Happaney K, Zelazo PD. Resistance to extinction: a measure of orbitofrontal function suitable for children? Brain Cogn. 2004;55:171–184. doi: 10.1016/S0278-2626(03)00270-7. [DOI] [PubMed] [Google Scholar]
- 84.Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 85.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1989;62:1175–1183. [PubMed] [Google Scholar]
- 86.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- 87.Suomi SJ, Harlow HF. The facts and functions of fear. In: Zuckerman M, Spielberger CD, editors. Emotions and Anxiety: New Concepts, Methods and Applications. Erlbaum; Hillsdale, NJ: 1976. pp. 3–34. [Google Scholar]
- 88.Raper J, Bachevalier J, Kazama AM. Blunted fear reactivity after neonatal amygdala and orbital frontal lesions in rhesus monkeys. Poster presentation at the Society for Neuroscience; Chicago. 2009. [Google Scholar]
- 89.Raper J, et al. Neonatal orbital frontal lesions alter basal and stress reactive cortisol levels in adult rhesus monkeys. Neuobiology of Stress Workshop 2010
- 90.Kazama A, Bachevalier J. Selective aspiration or neurotoxic lesions of orbital frontal areas 11 & 13 spared monkeys’ performance on the object discrimination reversal task. Poster presentation for Society of Neuroscience Meeting; Atlanta GA. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]