Fig. 5.
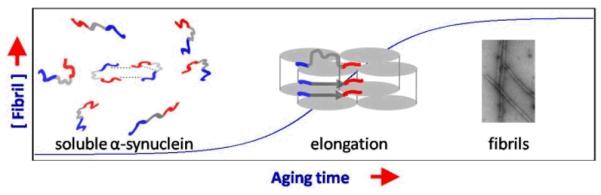
Schematic representation for a proposed aggregation mechanism of α-syn. Soluble α-syn ensemble is consisted of conformers that are heterogeneous, dynamic, and interchanging. Various long range and transient inter/intra-molecular interactions in α-syn have been characterized. The highly acidic C-terminal tail has been suggested to play a key role in modulating α-syn solution conformation. Due to constraints arising from existing long-range interactions in the soluble α-syn, local structural rearrangement at the N-and C-terminus is expected to occur in order to favor α-syn forming a cross β-fold in its amyloid state. Hence, the N- and C-termini experience early environmental changes preceding residues near the amyloid core during the elongation phase.
