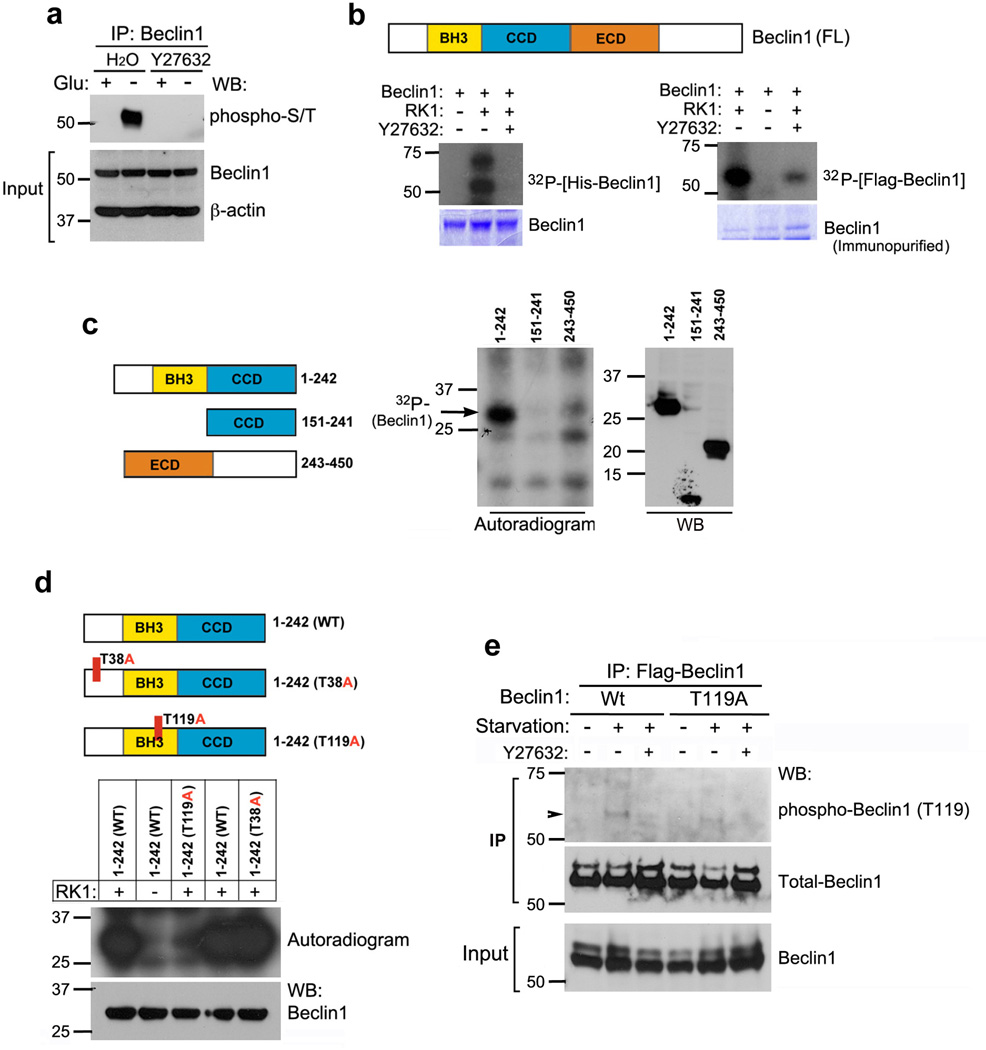
Figure 4. Metabolic stress induces Beclin1 phosphorylation by ROCK1.
(a) Inhibition of ROCK1 kinase activity prevents metabolic stress-mediated phosphorylation of Beclin1. HeLa cells left untreated or treated with a ROCK1 inhibitor, Y27632 (10 µM) were starved of glucose (4 h) as indicated, and endogenous Beclin1 was immunoprecipitated and blotted against phospho-Ser/Thr antibody. Whole cell lysates were run as inputs for Beclin1 and β-actin. (b) Phosphorylation of Beclin1 by ROCK1. Recombinant ROCK1 was used for in vitro kinase assay in the presence or absence of Y27632, using recombinant His-Beclin1 (left) or Flag-Beclin1 immunopurified from transfected HeLa cells (right). Proteins were resolved by SDS-PAGE; phosphorylated protein was visualized with autoradiography, and Beclin1 by Coomassie staining. (c) Identification of ROCK1-mediated phosphorylation domains in Beclin1. The indicated Flag-Beclin1 fragments were purified from HeLa cells and used as substrates for in vitro ROCK1 kinase assay.32P-autoradiogram (center) analyzed phosphorylation and western blotting (right) determined protein levels. Schematic representation of Beclin1 domain structure and deletion constructs are shown (left). (d) Identification of the phosphorylation site on Beclin1. Representation of point mutations in the different domains of Beclin1 are shown (left). In vitro kinase assay using Flag-Beclin1 WT and mutants (T38A and T119A), immunoprecipitated from transfected HeLa cells, as substrate and recombinant ROCK1 was performed. Phosphorylation was detected by32P-autoradiogram, and Flag-Beclin1 levels were examined by western blotting. (e) Flag-Beclin1 Wt or T119A transfected HeLa cells were incubated in glucose-rich or nutrient free (HBSS) media, in the presence or absence of ROCK1 inhibitor Y27632. Total cell extracts were used for immunoprecipitation using Flag agarose. Eluted protein was analyzed by western blotting against phospho-T119 (Beclin) antibody and Beclin1. Input for Beclin1 was run on 7.5% gel and immunoblotted.