Table 5.
Comparison of Pd/Ir-catalyzed C–H phenylation with Ph2I+ vs Pd/Rucatalyzed C–H phenylation with PhN2+.
| Substrate | Product | Yield (%) with Ph2I +[a,b] | Yield (%)with PhN2+[a,c] |
|---|---|---|---|
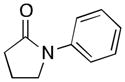 (1) |
1a | 89 | 91 |
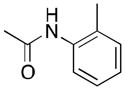 (3) |
3a | 69[d] | 89 |
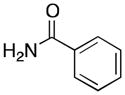 (5) |
5a | 54 | 25 |
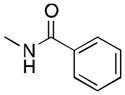 (6) |
6a | 52 | 38 |
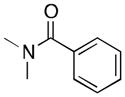 (7) |
7a | 11 | 8 |
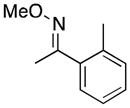 (10) |
10a | 52 | 23 |
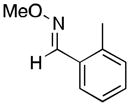 (11) |
11a | 63 | 68 |
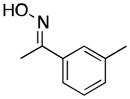 (17) |
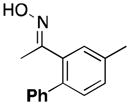
|
<1[e] | 66 |
GC calibrated yield.
General conditions: substrate (1 equiv), [Ph2I]OTf (2 equiv), Pd(NO3)2 (0.10 equiv), Ir(ppy)2(dtbbpy)PF6 (0.05 equiv), MeOH (0.2 M in substrate), 26 W lightbulb, 15 h, r.t., degassed by sparging with N2.
General conditions: substrate (1 equiv), [PhN2]BF4 (4 equiv), Pd(OAc)2 (0.10 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), MeOH (0.1 M in substrate), 26 W lightbulb, r.t., 15 h, degassed by sparging with N2.
[Ph2I]BF4 was the oxidant.
Product 17a was not detected by GC, and only traces amounts of 17 and 3′-methylacetophenone were formed.
