Abstract
Objective:
Early detection of smooth surface lesions is important for appropriate management and monitoring of dental caries. The purpose of this in vitro study was to evaluate the efficacy of laser fluorescence to detect demineralization and remineralization of smooth enamel surfaces.
Materials and Methods:
In this in vitro study, 132 enamel blocks of semi-impacted human third molars were obtained; artificial caries lesions were induced and they were submitted to the pH-cycling process to create remineralization. Superficial microhardness (SMH) and laser fluorescence (LF) analysis were performed at baseline, after demineralization, and remineralization processes. The data were analyzed by Statistical Package for Social Sciences (SPSS)-16 using analysis of variance (ANOVA), Paired samples t-test, and Pearson's correlation test.
Results:
There was a significant difference between SMH values at baseline, after demineralization and after remineralization. Also, a statistically significant difference was observed between LF values in these three stages. The LF values increased after demineralization and then decreased after remineralization, and the SMH values decreased after demineralization and increased after remineralization. There was an inverse relationship between SMH and LF only at baseline and after demineralization, but not after remineralization.
Conclusion:
The results showed that LF is an appropriate method for detection of demineralization in an in vitro condition in smooth enamel lesions, but it was not so efficient in the detection of remineralization.
Keywords: Demineralization, DIAGNOdent, enamel, laser fluorescence, remineralization, SMH, smooth surfaces
INTRODUCTION
For caries control, it is necessary to detect caries’ lesions in an early stage of histological changes.[1] Visual, tactile, and radiographic approaches are just the basic and most commonly used methods for caries detection. But these methods are subjective, with a low reproducibility.[2]
Some light-based methods are used for caries detection, such as conventional radiography, micro radiography, Digital Imaging Fiber Optic Transillumination Imaging (DIFOTI), quantitative light-induced fluorescence (QLF), and laser-induced fluorescence (DIAGNOdent).[3]
Laser fluorescence (LF) is a method introduced for early diagnosis of the dental caries. It is useful for early detection of hidden caries in non-cavitated teeth through a non-invasive method. It emits infrared light (655 nm), that can be absorbed by organic and inorganic tooth materials, and the process of remitted fluorescence shows various scales between 0 and 99. The value of 20 or 25 and higher indicates caries lesion, higher the scale deeper the caries invasion.[4,5,6,7]
The exact mechanism of DIAGNOdent is still not clear, but two theories exist: First, when the red light meets a change in tooth tissue, such as porosity due to demineralization or hypomineralization, it stimulates fluorescent light of a different wavelength. Second, some bacterial metabolites such as porphyrines (proto-porphyrine, meso-porphyrine, or proporphyrin), result in the red fluorescence of carious teeth.[8,9]
Although several studies have been conducted to find the efficacy and validity of DIAGNOdent for detection of occlusal and smooth surfaces caries,[10,11,12,13] little evidence is available about its efficacy in monitoring of remineralization in incipient caries.[11]
Diniz et al., (2009) carried out a study about the efficacy of laser fluorescence in detection ofdemineralization and remineralization in bovine teeth. They concluded that this device was not efficient in monitoring of demineralization and remineralization in enamel smooth surfaces.[11] Mendes (2003) in an in vivo study found that laser fluorescence was not favorable for monitoring of remineralization in primary teeth as well.[14]
In contrary, Shi et al., (2001) have suggested LF to be efficacious for assessment of smooth-surface caries.[10] These controversies are probably due to different methods used in each study.
Several qualitative methods are used to determine changes in mineral materials of enamel during caries process, such as Transverse microradiography, polarized light microscopy, and microhardness measurements (SMH). SMH is a simple and harmless method that gives some information about surface mineral changes, but not about mineral changes in the body of caries lesion.[15,16,17]
The aim of this in vitro study was to evaluate the efficacy of DIAGNOdent to detect demineralization and remineralization of smooth enamel surfaces.
MATERIALS AND METHODS
In this laboratory trial, 54 extracted semi-erupted upper and lower third molars with no clinical signs of caries or white spot, were selected. Two hundred sixteen enamel blocks [four blocks (4 × 4 mm) for each tooth] were obtained by mesiodistal and buccolingual sections from each tooth with disc and mandrel (D and Z, Germany). The specimens were cleaned and washed with water, then stored in distilled water at room temperature. Each block was mounted in epoxy resin for a suitable surface microhardness analysis. Then the specimens were polished serially by Diamondpro (FGM Dental Products, Joinville, Brazil).
The microhardness analysis was done by microhardness tester using Vicker diamond (COOPA, MIH, Iran) under a 50 g load for 10 seconds.
From 216 blocks, only those with hardness of 338.02 ± 30.31 VMHn (Vicker Microhardness No) were selected for artificial caries lesion induction and the others were excluded from the study, due to their high variable microhardness values.
A laser fluorescence device (LF2190, DIAGNOdent, Kavo, Biberach, Germany) with probe tip B was used in our study. Two operators read LF to ensure validity of the observation and the inter-observer agreement was determined before the assessment.
The device was calibrated against a porcelain standard before reading and after testing each 5-6 blocks. Then each block was dried with gauze. The probe tip B was kept perpendicular to the tooth surface.
The maximum LF values were recorded for each block. After reading each block three times by each operator in a 1-week period, the mean values were obtained. Then the blocks were immersed in a 12 ml demineralization solution made of 1% sodium carboxymethyl cellulose in 0.1 M of lactic acid (containing 3 mM of calcium and 1.8 mM of phosphate, pH = 4).[18]
The blocks were placed in an incubator for 36 hours at 37°C. The surface hardness test was done again after demineralization, and mean and standard deviation (SD) were calculated and 132 blocks with SMH 67.57 ± 24.14 were selected for pH-cycling process.[11] The other 40 blocks were excluded from the study due to large differences in the mean and standard deviation after demineralization.
PH-cycling method was done as follows: A fluoride dentifrice containing 1450 ppm (Stannous Fluoride, Crest 7 complete expert, Germany) was applied. PH-cycling process includes 1 minute soaking in 100 ml 33% (w/v) dentifrice/water slurries four times per day (8:00 A.M., 10 A.M., 2:30 P.M., and 4:30 P.M.) to stimulate tooth brushing exposure [Paes Leme[19].
Then the blocks were washed with deionized water and immersed in 4 ml of artificial saliva (BioXtra Alcohol free Mouthwash, Biohelp, Belgium) at 37°C.
Thereafter, they were immersed in 12 ml of a demineralization solution from 12 A.M. to 2 P.M. to mimic the acidic conditions of oral cavity. The continuous demineralization and remineralization cycles were done for 6 days. The solution was replaced after 3 days.[10] After this stage, LF reading and SMH were recorded again for each block and the results were compared to the results of baseline and after demineralization.
Statistical analysis
The data were analyzed by Statistical Package for Social Sciences (SPSS)-16 using analysis of variance (ANOVA), Paired samples t-test, and Pearson's correlation test.
RESULTS
To evaluate the inter-and intra-examiner agreement, the term “intra-class correlation (ICC)” was used. ICC results below 0.4 was considered as poor, 0.40-0.59 as fair, 0.60-0.75 as good, and above 0.75 as excellent.[11]
The results showed no statistically significant difference in mean DIAGNOdent readings between two operators at baseline (P = 0.699), after demineralization (P = 0.774), and after remineralization phases (P = 0.161). According to Pearson's correlation test, the inter-examiner correlation was 0.94, 0.99, and 0.97 at baseline, after demineralization phase, and after remineralization phase, respectively.
Table 1 shows the mean values of SMH and LF readings at three different times, and Table 2 shows two by two comparisons of SMH and LF readings in three stages.
Table 1.
Mean SMH and LF readings of enamel surfaces in three stages
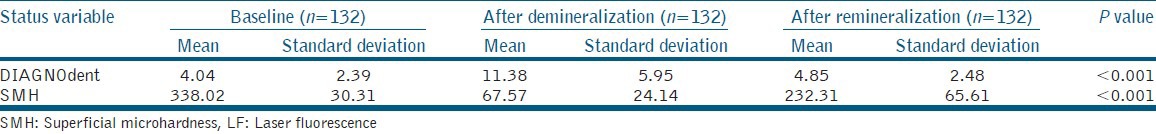
Table 2.
Two by two comparisons of mean SMH and LF readings of enamel surfaces in three stages
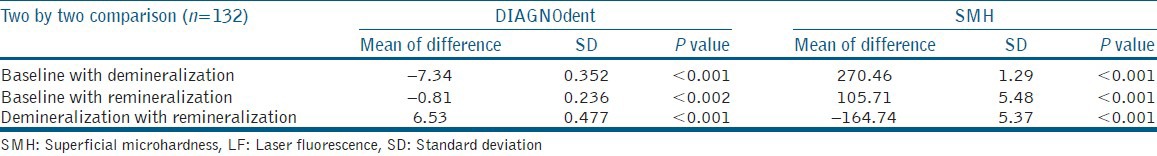
The comparison between baseline and demineralization, between baseline and remineralization, and between demineralization and remineralization values showed significant difference (P < 0.001, P < 0.002, and P < 0.001, respectively).
The results showed that microhardness was significantly decreased after demineralization, and was significantly increased after remineralization that was still lower than baseline.
The distribution of LF values according to SMH is shown in Figures 1, 2, and 3. Figure 1 shows the inverse linear relationship between DIAGNOdent and microhardness values at baseline. Also the same result was found after demineralization [Figure 2], but this inverse linear relationship was not observed after remineralization [Figure 3].
Figure 1.
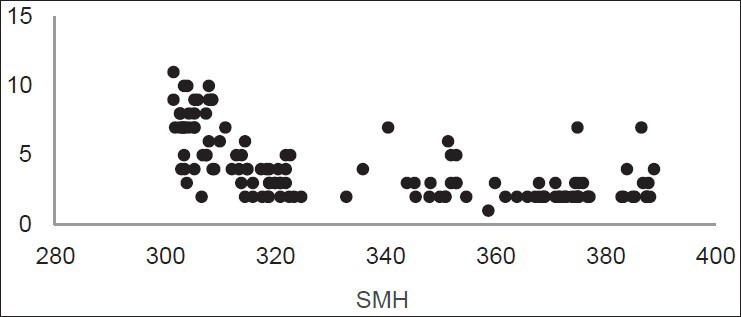
Distribution of LF readings according to SMH at baseline
Figure 2.
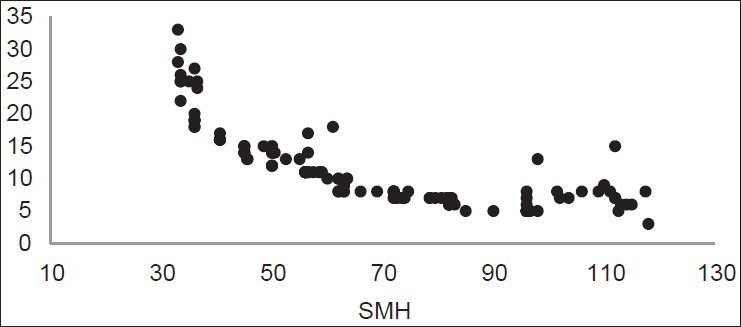
Distribution of LF readings according to SMH after demineralization
Figure 3.
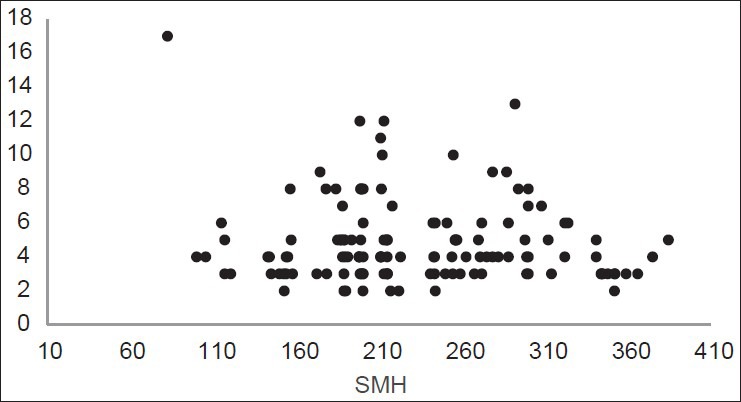
Distribution of LF readings according to SMH after remineralization
DISCUSSION
Three major steps in management of dental caries are prevention, control, and treatment. So, it requires appropriate detection of caries lesion, especially in its early stage, and monitoring its progression.[20]
Optical properties have been shown to be useful in determination of mineral loss in enamel tissue.[21] LF device was introduced for occlusal and smooth surface caries detection.[4] It can record the difference in the fluorescing capacity of the sound tooth and the carious lesion. Changes in some properties of mineral components, such as reflection, transmission, and color absorption in demineralized teeth, affect LF reading and so the value is different from healthy teeth, which helps in detection of caries.[11,21]
In current study, the inter-examiner agreement was between 0.94 and 0.99. Kuhnish et al., in an in vitro study to assess the reliability of DIAGNOdent measurement,[22] and Aljehani et al., found the values of inter-examiner agreement to be 0.93-0.98 and 0.91-0.99, respectively.[6] These two studies were in agreement with our study that showed excellent ICC. Diniz found ICC to be between 0.51 and 0.61, that was different from our results.[11] This is probably due to technical errors, so it is necessary to train the operators before experiment.
Previous studies conducted to detect demineralization and remineralization with DIAGNOdent, were either experimental[11,14,23] or in situ ones,[24,25,26] and one study considered both experimental and in situ conditions.[1]
In the current research, the comparison of the results of LF at baseline and after demineralization showed a significant difference. Diniz et al.,[11] Shi et al.,[10] and Mendes et al.,[13] reported no statistically significant difference that can be related to some misdiagnoses, such as inadequate diagnosis of minor changes in inorganic components by LF. It may probably be due to differences in demineralization solutions, pH-cycling procedures, and artificial solutions.
The LF values were increased after demineralization and then decreased after remineralization, but they were still higher from baseline, this can be due to some factors such as operator errors.
We also used SMH as a standard and results showed a significant decrease in microhardness after demineralization and an increase after remineralization.
Our results about increased LF values after demineralization were consistent with the results of Mendes and Nicolau. They attributed this increase to enhanced porosity in enamel surface due to decrease in overall mineral content.[13]
Furthermore, decrease in values after remineralization was in agreement with Diniz et al., study and can be due to a decrease in porosity of enamel surface.[11]
The increased mean LF reading after pH-cycling than baseline was similar to Gokalp and Baseren's results[27] and inconsistent with Mendes et al., study.[13] This can be explained by the difference in samples (primary or permanent teeth).
We found LF as a useful device for detection of demineralization that is in contrast to Silva et al., They found no changes in LF values of non-cavitated teeth in an in vivo condition and concluded that it was not a suitable method for this purpose.[14]
According to current study, DIAGNOdent was not useful for assessment of remineralization. This result is similar to Mendes et al.,[14] Diniz et al.,[11] and Jablonski-Momeni et al.,[23] Silva et al.,[25] and Guimaraes et al.,[24] but it was contrary to Spiguel et al.,[1] and Celiberti et al.,[26]
Some factors can produce different results in different studies, such as different toothpastes components or artificial solutions, different maintenance solutions (thymol, formalin, chloramines or saline or distilled water), different cut-off limits of manufacturers for LF,[28] type of the study (in vivo or in vitro), condition of the study and sample selection (bovine, hypomineralized, primary, or permanent teeth).
There are some differences between clinical and laboratory conditions, such as presence of bacteria in clinical studies, absence of some enzymes in laboratory trials e.g., monofluorophosphate (MSP) that has a hydrolyzing effect in oral cavity and can influence the pH-cycling process.[29]
Some components of toothpastes remain in pits and fissures and cause increased LF reading. Also, in most in vitro studies, the results of LF readings were lower than in vivo ones.[11,29]
We found increased DIAGNOdent and decreased microhardness values after demineralization that followed by a decrease in DIAGNOdent and an increase in microhardness values after remineralization, but it did not reach the baseline value. These increasing or decreasing trends were in consistent with Diniz[11] and Spiguel[1] studies, but the values of the current study were higher than Diniz, probably due to the usage of human teeth instead of bovine teeth, different storage solutions, different demineralization and remineralization solutions, and different pH-cycling processes.
CONCLUSION
Four major conclusions of this research were:
The DIAGNOdent values increased after demineralization and then decreased after remineralization.
The microhardeness values decreased after demineralization and increased after remineralization.
There was an inverse linear relationship between DIAGNOdent and microhardness values at baseline and after demineralization, but not after remineralization.
DIAGNOdent is an appropriate device for detection of demineralization processes in smooth enamel lesions, but it was not so efficient in the detection of remineralization in an in vitro condition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Spiguel MH, Tovo MF, Kramer PF, Franco KS, Alves KM, Delbem AC. Evaluation of laser fluorescence in the monitoring of the initial stage of the de-/ramineralization process: An in vitro and in situ study. Caries Res. 2009;43:302–7. doi: 10.1159/000218094. [DOI] [PubMed] [Google Scholar]
- 2.Pretty IA. Caries detection and diagnosis: Novel technologies. J Dent. 2006;34:727–39. doi: 10.1016/j.jdent.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Kishen A, Shrestha A, Rafigue A. Fiber optic backscatter spectroscopic sensor to monitor enamel demineralization and remineralization in vitro. J Conserv Dent. 2008;11:63–70. doi: 10.4103/0972-0707.44053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendes FM, Siqueira WL, Mazzitelli JF, Pinheiro SL, Bengtson AL. Performance of DIAGNOdent for detection and quantification of smooth-surface caries in primary teeth. J Dent. 2005;33:79–84. doi: 10.1016/j.jdent.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.McDonald RE, Avery DR, Stookey GK, Chin JR, Kowolik JE. Dental caries in the child and adolescent. In: Dean JA, Avery DR, McDonald RE, editors. Dentistry for the Child and Adolescent. Missouri: Mosby; 2011. p. 187. [Google Scholar]
- 6.Aljehani A, Bamzahim M, Yousif MA, Shi XQ. In vivo reliability of an infrared fluorescence method for quantification of carious lesions in orthodontic patients. Oral Health Prev Dent. 2006;4:145–50. [PubMed] [Google Scholar]
- 7.Hibst R, Paulus R, Lussi A. Detection of occlusal caries by laser fluorescence: Basic and clinical investigations. Med Laser Appl. 2001;16:205–13. [Google Scholar]
- 8.Buchalla W, Attin T, Niedmann Y, Niedmann PD, Lennon AM. Porphyrins are the cause of red fluorescence of carious dentin: Verified by gradient reversed-phase HPLC. Caries Res. 2008;42:223. [Google Scholar]
- 9.Amaechi BT. Emerging technologies for diagnosis of dental caries: The road so far. J Appl Phys. 2009;105 102047. [Google Scholar]
- 10.Shi XQ, Tranaeus S, Angmar-Mansson B. Comparison of QLF and DIAGNOdent for quantification of smooth surface caries. Caries Res. 2001;35:21–6. doi: 10.1159/000047426. [DOI] [PubMed] [Google Scholar]
- 11.Diniz MB, Leme AF, Cardoso Kde S, Rodrigues Jde A, Corderio Rde C. The efficacy of laser fluorescence to detect in vitro demineralization and remineralization of smooth enamel surfaces. Photomed Laser Surg. 2009;27:57–61. doi: 10.1089/pho.2007.2230. [DOI] [PubMed] [Google Scholar]
- 12.Pinelli C, Campos Serra M, de Castro Monteiro Loffredo L. Validity and reproducibility of a laser fluorescence system for detecting the activity of white-spot lesions on free smooth surfaces in vivo. Caries Res. 2002;36:19–24. doi: 10.1159/000057585. [DOI] [PubMed] [Google Scholar]
- 13.Mendes FM, Nicolau J. Utilization of laser fluorescence to monitor caries lesions development in primary teeth. J Dent Child (Chic) 2004;71:139–42. [PubMed] [Google Scholar]
- 14.Mendes FM, Nicolau J, Duarte DA. Evaluation of the effectiveness of laser fluorescence in monitoring in vitro remineralization of incipient caries lesions in primary teeth. Caries Res. 2003;35:442–4. doi: 10.1159/000073397. [DOI] [PubMed] [Google Scholar]
- 15.Doerner MF, Nix WD. A method for interpreting data from depth-sensing indentation measurement. J Mater Res. 1986;1:601–9. [Google Scholar]
- 16.Rehder Neto FC, Maeda FA, Turssi CP, Serra MC. Potential agents to control enamel caries-like lesions. J Dent. 2009;37:786–90. doi: 10.1016/j.jdent.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Huysmans MC, Longbottom C. The challenges of validating diagnostic methods and selecting appropriate gold standards. J Dent Res. 2004;83:C48–52. doi: 10.1177/154405910408301s10. [DOI] [PubMed] [Google Scholar]
- 18.Pai D, Bhat SS, Taranath A, Sargod S, Pai VM. Use of laser fluorescence and scanning electron microscope to evaluate remineralization of incipient enamel lesions remineralized by topical application of casein peptide amorphous calcium phosphate (CPP-ACP) containing cream. J Clin Pediatr Dent. 2008;32:201–6. doi: 10.17796/jcpd.32.3.d083470201h58m13. [DOI] [PubMed] [Google Scholar]
- 19.Paes Leme AF, Tabchoury CP, Zero DT, Cury JA. Effect of fluoridated dentifrice and acidulated phosphate fluoride application on early artificial carious lesions. Am J Dent. 2003;16:91–5. [PubMed] [Google Scholar]
- 20.Tranaeus S, Shi XQ, Angmar-Mansson B. Caries risk assessment: Method available to clinicians for caries detection. Community Dent Oral Epidemiol. 2005;33:265–73. doi: 10.1111/j.1600-0528.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 21.Craounanidy U, Sathyanarayanan R. Dental caries: A complete changeover (Part II)-Changeover in the diagnosis and prognosis. J Conserv Dent. 2009;12:87–100. doi: 10.4103/0972-0707.57631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhnisch J, Ziehe A, Bradstadt A, Heinrich-Weltzein R. An in vitro study of the reliability of DIAGNOdent measurements. J Oral Rehabil. 2004;31:895–9. doi: 10.1111/j.1365-2842.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 23.Jablonski-Momeni A, Ricketts DN, Rolfsen S, Stoll R, Heinzel-Gutenbrunner M, Stachniss V, et al. Performance of laser fluorescence at tooth surface and histological section. Lasers Med Sci. 2011;26:171–8. doi: 10.1007/s10103-010-0768-y. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes AR, Vieira RD, Minamisako MC, Modesto A, Cury JA. DIAGNOdent vs. SMH in non-cavitated caries lesions monitoring. IADR/AADR/CADR 83rd General Session March 9-12 2005 [Google Scholar]
- 25.Silva BB, Severo NB, Maltz M. Validity of diode laser to monitor caries lesions in pits and fissures. J Dent. 2007;35:679–82. doi: 10.1016/j.jdent.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Celiberti P, Leamari VM, Imparato JC, Braga MM, Mendes FM. In vitro ability of a laser fluorescence device in quantifying approximal caries lesions in primary molars. J Dent. 2010;38:666–70. doi: 10.1016/j.jdent.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Gokalp S, Baseren M. Use of laser fluorescence in monitoring the durability and cariostatic effects of fluoride and chlorhexidine varnishes on occlusal caries: A clinical study. Quintessence Int. 2005;36:183–9. [PubMed] [Google Scholar]
- 28.Diniz MB, Rodrigues JA, de Paula AB, Cordeiro Rde C. In vivo evaluation of laser fluorescence performance using different cut-off limits for occlusal caries detection. Lasers Med Sci. 2009;24:295–300. doi: 10.1007/s10103-008-0547-1. [DOI] [PubMed] [Google Scholar]
- 29.Buzalaf MA, Hannas AR, Magalhaes AC, Rios D, Hornorio HM, Delbem AC. PH-cycling models for in vitro evaluation of the efficacy of fluoridated dentrifrices for caries control: Strengths and limitations. J Appl Oral Sci. 2010;18:316–34. doi: 10.1590/S1678-77572010000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]


