Figure 2. The standard study design for proof-of-concept trials of novel interventions in new-onset T1D.
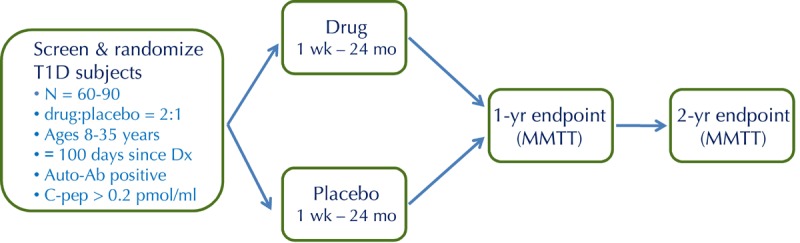
This is a randomized, placebo-controlled, double-blind phase 2 trial, with 2:1 randomization (drug to placebo). Key inclusion criteria are as shown, including ≤100 days since diagnosis (Dx). The primary endpoint, generally at 1 year, is the change from baseline in C-peptide area under the curve (AUC) following a mixed-meal tolerance test (MMTT).
