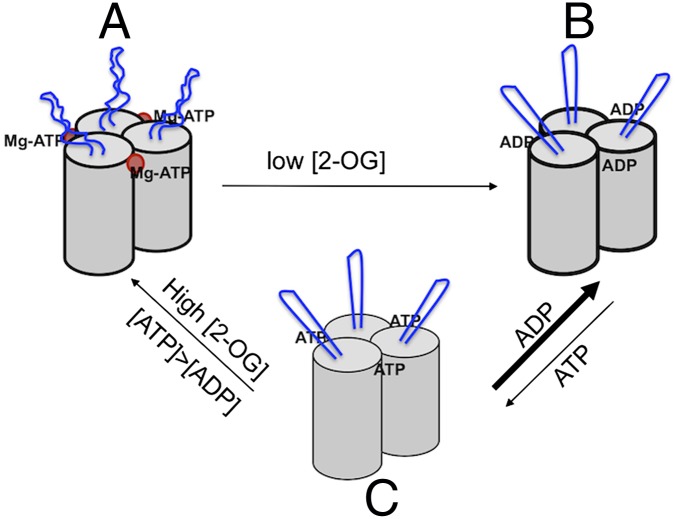
Fig. 1.
PII is a homotrimer, each subunit with a cylindrical core structure and antenna-like T-loop (in blue) extending out from the core. (A) Under nitrogen limitation, Mg-ATP and 2-OG (red dot) bind at proximity at the cleft between each subunit; the presence of 2-OG inhibits the hydrolysis of ATP, and the T-loops adopt a flexible conformation. (B) When nitrogen supply increases, 2-OG is consumed for ammonium assimilation and its level drops; Mg-ATP is hydrolyzed to ADP and the T-loops become more rigid. ADP inhibits ATP binding under low 2-OG levels. (C) High 2-OG and ATP concentrations favor the exchange of ADP to ATP, and PII is reloaded with Mg-ATP and 2-OG.